stat3靶向环寡核苷酸的安全性和有效性:从小鼠模型到口腔癌宠物猫的1期临床试验
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
STAT3是一种致癌转录因子,可激活癌细胞信号传导并诱导免疫抑制环境,使其成为一个有吸引力的治疗靶点。转录因子是极具挑战性的靶标,目前还没有食品和药物管理局批准的STAT3抑制剂。我们之前报道了在头颈癌鳞状细胞癌(HNSCC)患者的0期试验中瘤内给予线性STAT3诱饵寡核苷酸的阳性药效学。在这里,我们描述了一种循环STAT3诱骗剂(CS3D)在免疫功能强的HNSCC小鼠模型中的抗肿瘤和免疫作用,以及CS3D在患有HNSCC的宠物猫的临床试验中的安全性和有效性。临床试验中的应答者(疾病控制率35%)与无应答者相比,在选定的外周血免疫参数以及肿瘤中PD-1表达升高方面存在显著差异。这些发现支持CS3D在HNSCC患者中的临床试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
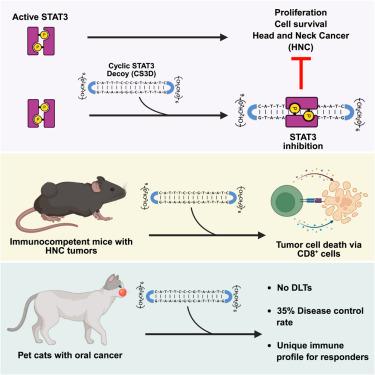
Safety and efficacy of a STAT3-targeted cyclic oligonucleotide: From murine models to a phase 1 clinical trial in pet cats with oral cancer
STAT3 is an oncogenic transcription factor that activates cancer cell signaling and induces an immunosuppressive immune environment, making it an attractive therapeutic target. Transcription factors are exceptionally challenging targets and there are no Food and Drug Administration-approved STAT3 inhibitors. We previously reported positive pharmacodynamics of a linear STAT3 decoy oligonucleotide administered intratumorally in a phase 0 trial in patients with head and neck cancer squamous cell carcinoma (HNSCC). Here, we describe the anti-tumor and immune effects of a systemically administered cyclic STAT3 decoy (CS3D) in immunocompetent HNSCC murine models and the safety and efficacy of CS3D in a clinical trial in pet cats with HNSCC. Responders in the clinical trial (35% disease control rate) showed significant differences in selected peripheral blood immune parameters as well as elevated PD-1 expression in the tumors compared with non-responders. These findings support a clinical trial of CS3D in HNSCC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


