nf1缺失的ER+乳腺癌对CDK4/6抑制剂的敏感性存在差异
IF 14.6
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
神经纤维蛋白/NF1是一种RAS(大鼠肉瘤病毒)gtpase激活蛋白和雌激素受体(ER)转录辅抑制因子。nf10低状态,通过拷贝数丢失或mRNA/蛋白低表达鉴定,与约20%的ER+/HER2−(人表皮生长因子受体2)早期乳腺癌的内分泌治疗耐药相关。因此,确定针对NF1low ER+/HER2 -乳腺癌的靶向治疗是一个优先事项。在这项研究中,ER+/HER2−乳腺癌的蛋白质基因组学分析表明,NF1low肿瘤表现出细胞周期蛋白依赖性激酶4/6 (CDK4/6)活性升高。在细胞系中,NF1缺失对CDK4活性有双重影响:首先,通过促进ER向CCND1 (cyclin D1)募集,从而增加CDK4 - cyclin D1复合物的形成;其次,通过激活C-RAF(快速加速纤维肉瘤),从而驱动CDK4激活环的磷酸化。临床前模型显示,与单独使用氟维司汀相比,NF1low ER+癌细胞对氟维司汀联合CDK4/6抑制剂更敏感,在体外诱导细胞死亡,在体内诱导ER+ NF1low患者来源的异种移植模型中持久的肿瘤消退。此外,NF1low ER+/HER2 -肿瘤对新辅助芳香酶抑制剂(AI)联合帕博西尼比单独新辅助AI更敏感,这可以通过抑制基于mrna的增殖评分来证明。这些数据与一个模型一致,即NF1缺失时ER和RAS的共激活可以驱动CDK4/6活性和内分泌治疗抵抗,但使nf10低ER+肿瘤易受CDK4/6抑制。临床级NF1诊断的发展应该优先考虑,以确定nf10低ER+乳腺癌是否应该接受调整的辅助治疗建议,以反映对CDK4/6抑制的反应性增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
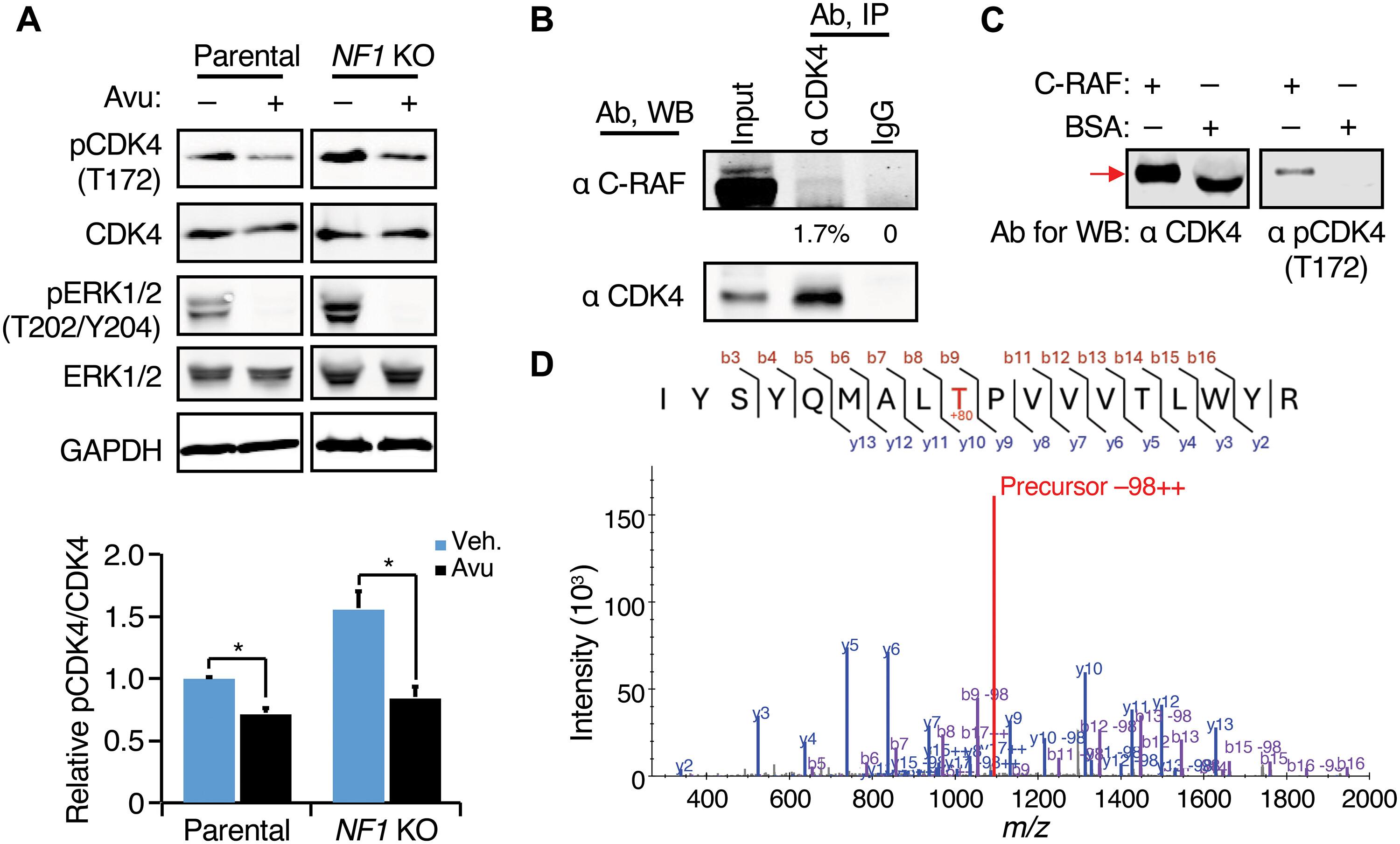
NF1-depleted ER+ breast cancers are differentially sensitive to CDK4/6 inhibitors
Neurofibromin/NF1 is a RAS (rat sarcoma virus) GTPase-activating protein and estrogen receptor (ER) transcriptional corepressor. NF1low status, identified by copy number loss or low mRNA/protein expression, is associated with endocrine therapy resistance in ~20% of ER+/HER2− (human epidermal growth factor receptor 2) early-stage breast cancers. The identification of targeted treatments for NF1low ER+/HER2− breast cancer is therefore a priority. In this study, proteogenomic analysis of ER+/HER2− breast cancer demonstrated that NF1low tumors exhibited elevated cyclin-dependent kinase 4/6 (CDK4/6) activity. In cell lines, NF1 deletion had a dual effect on CDK4 activity: first, by promoting ER recruitment to CCND1 (cyclin D1), thereby increasing CDK4–cyclin D1 complex formation, and second, by activating C-RAF (rapidly accelerated fibrosarcoma), which drove phosphorylation of the CDK4 activation loop. Preclinical modeling demonstrated that NF1low ER+ cancer cells were more sensitive to fulvestrant combined with a CDK4/6 inhibitor versus fulvestrant alone, with the induction of cell death in vitro and durable tumor regressions in ER+ NF1low patient-derived xenograft models in vivo. Furthermore, NF1low ER+/HER2− tumors were more sensitive to neoadjuvant aromatase inhibitor (AI) plus palbociclib than to neoadjuvant AI alone, as indicated by suppression of mRNA-based proliferation scores. These data are consistent with a model whereby ER and RAS coactivation upon NF1 loss can drive CDK4/6 activity and endocrine therapy resistance but renders NF1low ER+ tumors susceptible to CDK4/6 inhibition. Development of clinical-grade NF1 diagnostics should be prioritized to determine whether NF1low ER+ breast cancers should receive adjusted adjuvant treatment recommendations that reflect increased responsiveness to CDK4/6 inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


