可见光下氧化石墨烯(GO)与过氧化氢(H2O2)自由基协同作用机理研究
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氧化石墨烯(GO)是一种很有前途的用于降解有机污染物的无金属光催化剂。特别是GO和H2O2表现出协同效应,显著提高了对有机污染物的降解效率。然而,活性氧(ROS)在GO和H2O2系统(GO/H2O2)中产生和转化的机制尚未见报道。在这项研究中,我们在可见光下制备了具有光催化活性的氧化石墨烯。当以活性红X-3B (X-3B)为污染物时,GO/H2O2的协同效率提高了85.56%。我们采用电子顺磁共振(EPR)和自由基探针对活性氧进行定性和定量分析。结果表明,氧化石墨烯、氧化石墨烯和氧化石墨烯/氧化石墨烯体系分别生成了·OH、·O2−和1O2。这种协同效应主要是由GO在可见光下产生的H2O2和h+反应驱动的。h+是GO与H2O2协同作用的发动机。研究了·OH、·O2−和1O2在GO、H2O2和GO/H2O2体系中的转化关系。降解过程中自由基主要为1O2和·O2−。考察了GO/H2O2体系的影响因素,包括GO浓度、H2O2浓度、pH值、X-3B浓度和常见水基质。值得注意的是,氧化石墨烯具有优异的耐久性和矿化能力,即使经过6次循环也能保持优异的光催化性能。此外,在其应用过程中,氧化石墨烯中的缺陷数量增加,从而提高了光催化性能。这项工作阐明了ROS在GO/H2O2中的潜在协同机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
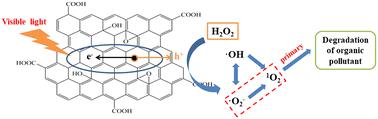
The synergistic mechanism of free radicals between graphene oxide (GO) and hydrogen peroxide (H2O2) under visible light†
Graphene oxide (GO) is a promising metal-free photocatalyst for the degradation of organic pollutants. In particular, GO and H2O2 exhibit a synergistic effect that significantly enhances the degradation efficiency of organic pollutants. However, the mechanism of reactive oxygen species (ROS) generation and transformation in the GO and H2O2 system (GO/H2O2) has not been reported. In this study, we prepared GO with photocatalytic activity under visible light. When targeting the reactive red X-3B (X-3B) as a pollutant, the synergistic efficiency of the GO/H2O2 was enhanced by 85.56%. We employed electron paramagnetic resonance (EPR) and free radical probe to conduct both qualitative and quantitative analyses of ROS. The results show that ·OH, ·O2− and 1O2 were produced in the GO, H2O2, and GO/H2O2 systems. The synergistic effect was mainly driven by the reaction of H2O2 and h+ produced by GO under visible light. h+ is the engine of the synergistic effect between GO and H2O2. The transformation relationships among ·OH, ·O2− and 1O2 in the GO, H2O2 and GO/H2O2 systems were investigated. The main free radicals in the degradation process were 1O2 and ·O2−. The influencing factors of the GO/H2O2 system were investigated, covering GO concentration, H2O2 concentration, pH value, X-3B concentration, and common water matrices. Notably, GO demonstrates excellent durability and mineralization capacity, maintaining superior photocatalytic performance even after 6 cycles. Moreover, during its application, the number of defects in GO increases, which improves the photocatalytic performance. This work clarified the underlying synergistic mechanism of ROS in GO/H2O2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


