P和CO2在Zn/P- zsm -5催化剂上对丙烷脱氢和芳构化的协同促进作用
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究通过DFT计算对Zn/P- zsm -5上丙烷脱氢芳构化反应机理进行了基础性的研究,并阐明了CO2和P对提高反应性能和催化剂稳定性的重要作用。确定了丙烷脱氢和丙烯芳构化的最佳能垒,分别为1.60 eV和1.44 eV。CO2的存在引入了新的和更容易的脱氢路线,从而降低了限速步骤的障碍,促进了反应。此外,CO2可以通过逆Boudouard反应消耗积碳,有利于延长催化剂寿命。在Zn/ZSM-5中添加P提高了活性位点的稳定性,增强了活性位点对水的抵抗力,增强了催化剂与反应物之间的相互作用,并通过产生新的非框架O位点诱导Zn- lewis位点的电子转移和电荷重新分配,从而改变了Zn- lewis位点的氧化态和酸度。CO2和P的协同促进作用为设计高效的沸石基轻烷烃脱氢芳构化催化剂提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
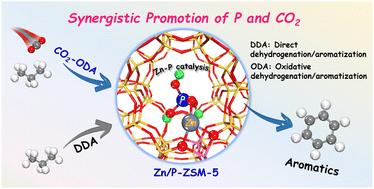
Identification of the synergistic promotion of P and CO2 on propane dehydrogenation and aromatization over the Zn/P-ZSM-5 catalyst
This study performed DFT calculations to provide fundamental insights into the reaction mechanism of propane dehydrogenation and aromatization over Zn/P-ZSM-5 and elucidated the important roles of CO2 and P in enhancing the reaction performance and catalyst stability. The rate-limiting steps for propane dehydrogenation and propene aromatization in the optimal energy pathways were identified, with energy barriers of 1.60 and 1.44 eV, respectively. The presence of CO2 introduced new and more facile dehydrogenation routes, thereby lowering the barriers of the rate-limiting steps and facilitating the reaction. Besides, CO2 could consume carbon deposits via the reverse Boudouard reaction, which is beneficial for extending the catalyst life. The addition of P to Zn/ZSM-5 improved the stability of active sites by strengthening their resistance towards water, enhanced the interactions between the catalyst and reactants, and induced electron transfer and charge redistribution at Zn-Lewis sites by creating new non-framework O sites, thus altering the oxidation state and acidity of the Zn-Lewis sites. The synergistic promotional effects of CO2 and P offer a promising strategy for designing efficient zeolite-based catalysts for the dehydrogenation and aromatization of light alkanes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


