阴毛皂苷A对溃疡性结肠炎的双重依赖作用:促进DDX5泛素化降解和Nrf2核易位抑制炎症
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
溃疡性结肠炎(UC)是一种慢性结肠炎症性疾病,具有复杂的临床症状。Pubescenoside A (PBA)是一种从短叶回青中提取的苯丙素,具有显著的抗炎作用;然而,PBA对UC的影响和潜在机制尚不清楚。因此,本研究的目的是通过体内和体外实验探讨PBA抗UC的潜在机制。阴毛皂苷A有效促进UC小鼠体重减轻,降低疾病活动指数(DAI)。在结肠形态方面,PBA改善结肠狭窄和缩短,减少肠黏膜溃疡,减少炎症细胞浸润,并倾向于使腺体排列正常化。此外,PBA还能有效抑制促炎因子。机制研究表明,PBA可直接靶向kelch样ECH相关蛋白1 (Keap1),进而促进Keap1与DEAD-box RNA解旋酶5 (DDX5)相互作用,增强DDX5的泛素化和降解,导致C-C基序趋化因子配体3 (CCL3)表达下调。此外,PBA破坏Keap1/Nrf2复合物,促进核因子红系2相关因子2 (Nrf2)进入核抑制核因子-κB (NF-κB)通路。重要的是,在Gpt-lgr5-creERT2 Keap1flox/flox小鼠中,PBA的抗uc作用减弱。我们的研究结果表明,PBA通过靶向Keap1来减轻UC,从而促进DDX5的泛素化降解,促进Nrf2的核进入。本文章由计算机程序翻译,如有差异,请以英文原文为准。
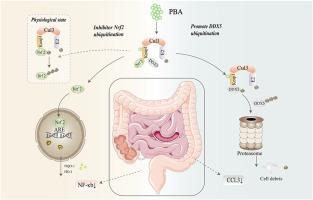
Dual Keap1-Dependent actions of pubescenoside A against ulcerative colitis: Promoting DDX5 ubiquitination degradation and Nrf2 nuclear translocation to suppress inflammation
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colon, characterized by a complex clinical syndrome. Pubescenoside A (PBA), a phenylpropanoid derived from Ilex pubescens, exhibits significant anti-inflammatory effects; however, the impact and underlying mechanism of PBA on UC remain unclear. Therefore, the aim of this study is to investigate the potential mechanism of PBA against UC using in vivo and in vitro experiments. Pubescenoside A effectively enhances weight loss in UC mice, decreases the disease activity index (DAI). Regarding colonic morphology, PBA ameliorates colorectal stenosis and shortening, reduces intestinal mucosal ulceration, diminishes inflammatory cell infiltration, and tends to normalize glandular arrangement. Furthermore, PBA effectively suppresses pro-inflammatory factors. Mechanism studies have shown that PBA can directly target Kelch-like ECH associated protein 1 (Keap1), subsequently promoting the interaction between Keap1 and DEAD-box RNA helicase 5 (DDX5) to enhance ubiquitination and degradation of DDX5, leading to a down-regulation of C-C motif chemokine ligand 3 (CCL3) expression. Additionally, PBA disrupts the Keap1/Nrf2 complex, facilitating Nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear entry to inhibit the Nuclear factor-κB (NF-κB) pathway. Importantly, in Gpt-lgr5-creERT2 Keap1flox/flox mice, the anti-UC effect of PBA was attenuated. Our results indicated that PBA mitigated UC by targeting Keap1, thereby promoting the ubiquitination degradation of DDX5 and facilitating the nuclear entry of Nrf2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


