基于金属配体协同增强策略的COX-2靶向ru -芳烃复合物用于乳腺癌治疗
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
由于其侵袭性和转移性的特点,三阴性乳腺癌(TNBC)一直是一个重大的治疗挑战。环氧化酶-2 (COX-2)在TNBC中经常过表达,与肿瘤进展有关,被认为是一种有效的治疗靶点。在这项研究中,我们报道了一种新的ru -芳烃配合物,Ru-tol,利用金属配体协同增强(MLSE)策略。以无毒前体叠氮化芳基钌和甲苯胺酸为原料合成了有毒钌醇。配合物Ru-tol不仅具有增强的抗增殖性能,而且保留了甲苯胺酸的COX-2抑制活性。Ru-tol可诱导细胞内活性氧(ROS)的产生,导致线粒体和细胞核损伤,最终导致细胞凋亡和自噬性死亡。此外,Ru-tol显著下调COX-2、基质金属蛋白酶(MMP)-2和MMP-9的表达,有效抑制细胞迁移和侵袭。Ru-tol在三维多细胞球体肿瘤(MCTS)中表现出良好的穿透能力和抗增殖作用,具有潜在的临床应用价值。这项工作不仅证明了MLSE策略在开发新型抗癌金属基药物方面的有效性,也为TNBC治疗提供了一个有希望的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
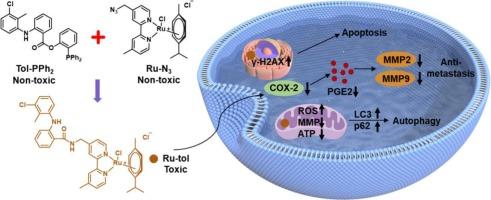
COX-2 targeting Ru-arene complexes from metal-ligand synergistic enhancement strategy for breast cancer therapy
Triple-negative breast cancer (TNBC) has been a significant therapeutic challenge, due to its aggressive and metastatic characteristics. Cyclooxygenase-2 (COX-2), which is frequently overexpressed in TNBC and implicated in tumor progression, is considered as a potent therapeutic target. In this study, we reported a novel Ru-arene complex, Ru-tol, utilizing a metal-ligand synergistic enhancement (MLSE) strategy. Toxic Ru-tol was synthesized from the non-toxic precursors aryl ruthenium azide and tolfenamic acid. Complex Ru-tol not only owned the enhanced antiproliferative performance, but also preserved the COX-2 inhibitory activity of tolfenamic acid. Ru-tol could induce the generation of intracellular reactive oxygen species (ROS), leading to the mitochondrial and nuclear damages, which ultimately results in the apoptotic and autophagic cell death. Moreover, Ru-tol significantly downregulated the expression of COX-2, matrix metalloproteinases (MMP)-2 and MMP-9, and effectively suppressed the cell migration and invasion. Ru-tol demonstrated supeior penetration capacity and antiproliferative efficacy in 3D multicellular tumor spheroids (MCTS), suggesting the potent clinical applications. This work not only demonstrated the efficiency of MLSE strategy for the development of novel anti-cancer metal-based drugs, but also presented a promising target for the TNBC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


