铬(III)的损失从混合铬(III),铁(III)血清转铁蛋白
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
三价铬已被证明在体内通过转铁蛋白(Tf)的内吞作用从血流转运到组织,转铁蛋白是血液中的主要铁转运蛋白。最近使用Cr(III)2-Tf的体外研究表明,在生理相关条件下,Cr(III)与Tf的结合以及Cr(III)2-Tf/Tf受体复合物中Cr(III)的损失是快速的。然而,血液中转铁蛋白的主要形式是单铁Tf。因此,考虑到血液中低浓度的Cr(III),通过内吞作用运输的含Cr(III)的转铁蛋白在血液中的形式是单色的,单色-Tf (Cr(III),Fe(III)-Tf)。由于Tf具有两个特定的金属结合位点,分别位于Tf的c端和n端叶中,因此可以形成两种形式的Cr(III),Fe(III)-Tf。本文首次研究了Cr(III)、Fe(III)-Tf两种形式中Cr(III)的损失。混合金属Tf损失Cr(III)的方式与Cr(III)2-Tf本身或Cr(III)2-Tf/Tf受体复合物损失Cr(III)的方式相似。本文章由计算机程序翻译,如有差异,请以英文原文为准。
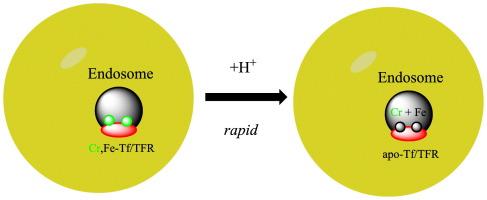
Loss of chromium(III) from mixed Cr(III),Fe(III) serum Transferrins
Trivalent chromium has been shown to be transported in vivo from the bloodstream to the tissues via endocytosis by transferrin (Tf), the major iron transport protein in the blood. Recent in vitro studies using Cr(III)2-Tf have shown that under physiologically relevant conditions, the binding of Cr(III) to Tf and the loss of Cr(III) from the Cr(III)2-Tf/Tf receptor complex are rapid. However, the major form of transferrin in the bloodstream is monoferric Tf. Thus, given the low concentrations of Cr(III) in the bloodstream, the form of Cr(III)-containing transferrin in the bloodstream that is transported via endocytosis is monochromic, monoferric-Tf (Cr(III),Fe(III)-Tf). Given that Tf has two specific metal-binding sites, one in both the C-terminal and the N-terminal lobes of Tf, two forms of Cr(III),Fe(III)-Tf can form. The loss of Cr(III) from both forms of Cr(III),Fe(III)-Tf have been examined for the first time. The mixed metal Tf's lose Cr(III) in similar fashions to Cr(III) losses from Cr(III)2-Tf itself or from the Cr(III)2-Tf/Tf receptor complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


