无细胞层形成的关键因素:红细胞压积和剪切速率梯度的实验研究
IF 2.7
4区 医学
Q2 PERIPHERAL VASCULAR DISEASE
引用次数: 0
摘要
血管壁附近无细胞层(CFL)的形成在微循环功能中起着关键作用,影响血液流变学、氧输送和内皮相互作用。虽然红细胞压积(Ht)是CFL厚度的一个公认的决定因素,但由于文献中相互矛盾的发现,剪切相关参数的影响仍然存在争议。在这项研究中,我们系统地量化了圆形玻璃微通道(25-50 μm直径)在不同红细胞比容水平(5 - 20%)、流速和悬浮介质(磷酸盐缓冲盐水和血浆)下的光学CFL厚度(δo)。采用高分辨率微流控成像和微粒子成像测速技术(μPIV)提取局部速度场,计算剪切速率梯度(∇γ ̄)。而不是将∇γ³作为一个强加的变量,我们将其表征为局部水动力环境的流动衍生描述符。在不同条件下,∇γ³与CFL厚度的相关性强于整体剪切速率。在PBS中,∇γ³的增加与CFL厚度的减小有关,这可能是由于剪切诱导色散增强所致。相反,在血浆中,较高的∇γ值促进了红细胞(RBC)聚集体的分解,恢复了流体动力升力,导致cfl变厚。这些趋势强调了在解释RBC行为时考虑悬浮介质和空间剪切变化的重要性。与先前的体外、体内和计算研究的比较表明,报道的CFL趋势的差异通常可以通过考虑聚集势和局部剪切速率梯度的差异来调和。这项工作为解释CFL动力学提供了一个统一的实验框架,并强调∇γ³是描述微循环中流动介导的红细胞再分布的一个有价值的参数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
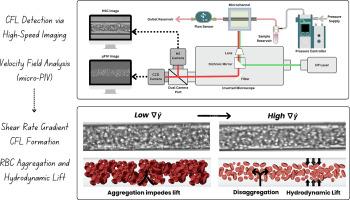
Key contributors to cell-free layer formation: An experimental investigation of hematocrit and shear rate gradient
The formation of the cell-free layer (CFL) near vessel walls plays a critical role in microcirculatory function, influencing blood rheology, oxygen delivery, and endothelial interactions. While hematocrit (Ht) is a well-established determinant of CFL thickness, the influence of shear-related parameters remains debated due to conflicting findings in the literature. In this study, we systematically quantified the optical CFL thickness () in circular glass microchannels (25–50 μm diameter) under varying hematocrit levels (5–20 %), flow rates, and suspension media (phosphate-buffered saline and plasma). High-resolution microfluidic imaging and micro-particle image velocimetry (μPIV) were used to extract local velocity fields and calculate shear rate gradients (∇).
Rather than treating ∇ as an imposed variable, we characterize it as a flow-derived descriptor of the local hydrodynamic environment. Across conditions, ∇ showed stronger correlations with CFL thickness than bulk shear rate. In PBS, increasing ∇ was associated with reduced CFL thickness, likely due to enhanced shear-induced dispersion. In contrast, in plasma, higher ∇ values promoted disaggregation of red blood cell (RBC) aggregates and restored hydrodynamic lift, resulting in thicker CFLs. These trends underscore the importance of considering both the suspension medium and spatial shear variations when interpreting RBC behavior.
Comparison with prior in vitro, in vivo, and computational studies suggests that discrepancies in reported CFL trends can often be reconciled by accounting for differences in aggregation potential and local shear rate gradients. This work provides a unified experimental framework for interpreting CFL dynamics and highlights ∇ as a valuable parameter for describing flow-mediated RBC redistribution in the microcirculation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microvascular research
医学-外周血管病
CiteScore
6.00
自引率
3.20%
发文量
158
审稿时长
43 days
期刊介绍:
Microvascular Research is dedicated to the dissemination of fundamental information related to the microvascular field. Full-length articles presenting the results of original research and brief communications are featured.
Research Areas include:
• Angiogenesis
• Biochemistry
• Bioengineering
• Biomathematics
• Biophysics
• Cancer
• Circulatory homeostasis
• Comparative physiology
• Drug delivery
• Neuropharmacology
• Microvascular pathology
• Rheology
• Tissue Engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


