转移性乳腺癌细胞通过ddx3 - drp1介导的线粒体可塑性易受脂肪酸氧化抑制
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
转移性肿瘤细胞在克服不同微环境障碍和在次要器官中生长方面表现出独特的代谢灵活性;因此,代谢脆弱性可能成为潜在的目标。据报道,线粒体的生物发生和动力学在播散性肿瘤细胞满足能量需求和代谢可塑性中起着至关重要的作用。然而,线粒体动力学促进肿瘤转移的具体分子机制尚不清楚。在此,我们发现转移性乳腺癌细胞在线粒体中表现出增加的脂质含量,并促进了向脂肪酸氧化的代谢转变(FAO)。粮农组织的增加伴随着线粒体裂变的促进。在机制上,我们发现DEAD-box多肽3,X-linked (DDX3)的上调促进了线粒体裂变并促进了FAO。抑制DDX3降低了转移性肿瘤细胞的FAO并引起线粒体氧化应激。此外,DDX3通过与周期蛋白依赖性激酶1 (CDK1)协同介导动力蛋白相关蛋白1 (DRP1)在S616位点的磷酸化。抑制DDX3-DRP1-CDK1轴可降低肿瘤干性和肿瘤转移。我们的研究结果表明,DDX3调节线粒体可塑性,驱动乳腺癌肿瘤转移的代谢适应。DDX3提供了一种潜在的诊断生物标志物和治疗脆弱性,通过它可以靶向癌症代谢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
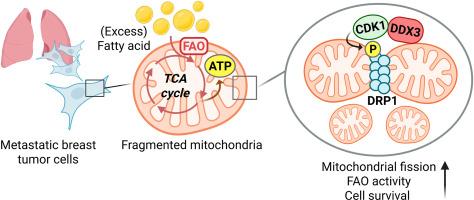
Metastatic breast cancer cells are vulnerable to fatty acid oxidation inhibition through DDX3-DRP1-mediated mitochondrial plasticity
Metastatic tumor cells exhibit distinct metabolic flexibility in overcoming different microenvironmental obstacles and thriving in a secondary organ; thus, metabolic vulnerabilities can potentially be targeted. It was reported that mitochondrial biogenesis and dynamics play crucial roles in disseminated tumor cells satisfying their energy demands and metabolic plasticity. However, the detailed molecular mechanism by which mitochondrial dynamics promotes tumor metastasis is still unclear. Herein, we identified that metastatic breast cancer cells exhibited increased lipid contents in mitochondria and promoted a metabolic shift towards fatty acid oxidation (FAO). The increased FAO was accompanied by promotion of mitochondrial fission. Mechanistically, we found that upregulation of DEAD-box polypeptide 3, X-linked (DDX3) promoted mitochondrial fission and facilitated FAO. Suppression of DDX3 diminished FAO and elicited mitochondrial oxidative stress in metastatic tumor cells. Moreover, DDX3 mediated dynamin-related protein 1 (DRP1) phosphorylation at S616 through collaborating with cyclin-dependent kinase 1 (CDK1). Inhibition of the DDX3-DRP1-CDK1 axis reduced cancer stemness properties and tumor metastasis. Our findings indicate that DDX3 modulates mitochondrial plasticity to drive metabolic adaptation in breast tumor metastasis. DDX3 provides a potential diagnostic biomarker and therapeutic vulnerability through which cancer metabolism can be targeted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


