脂肪性肝炎改变淋巴细胞的细胞毒性和定位,加速结直肠肝转移
IF 7.7
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
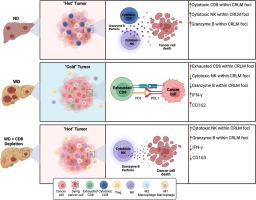
背景和目的肝脏是肿瘤最常见的远处转移部位。代谢功能障碍相关脂肪性肝病(MAFLD)是世界范围内最常见的肝脏疾病,显著增加结直肠癌(CRC)患者肝转移的风险。我们的目的是阐明肝脏免疫细胞在MAFLD代谢应激反应中的改变及其对早期结直肠癌肝转移(CRLM)的影响。方法采用高脂日粮(HFD)和西式日粮(WD)分别制备mald和MASH。通过脾内注射MC38癌细胞建立CRLM模型。利用单细胞RNA测序(scRNA-seq)、RT-PCR和免疫组织学研究肝脏免疫细胞的组成、表型和定位。结果两种饮食都显著增加了CRLM的建立,而只有WD改变了肝脏炎症。wd促进IL-10和TGF-β1的升高,这是一种抗炎细胞因子,抑制细胞毒性CD8+ T细胞和NK细胞,并支持免疫抑制环境。虽然MASH导致肝脏CD8+和NK细胞的存在增加,但它们对转移灶的浸润减少,并与细胞毒性标记物的表达减少有关。在我们的小鼠MASH模型中,CD8+ t细胞耗损减少了CRLM病灶的数量,这伴随着IFN-γ-相关细胞因子的减少和表达颗粒酶- b的NK细胞浸润的显著增加,最终增强了细胞毒杀伤能力。结论饮食诱导的免疫变化对CRLM的建立和发展有重要影响。这表明免疫细胞在肝转移中的共定位显著影响其功能,突出了平衡免疫衰竭和激活的潜在治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Steatohepatitis alters lymphocytes cytotoxicity and localization, accelerating colorectal liver metastases
Background & Aims
The liver is the most common site for distant metastasis. Metabolic dysfunction-associated fatty liver disease (MAFLD) is the most common liver disease worldwide and significantly increases the risk of liver metastasis in colorectal cancer (CRC) patients. We aimed to elucidate hepatic immune cells alterations in response to the metabolic stress in MAFLD and their influence on the early stages of CRC liver metastasis (CRLM).
Methods
High-fat diet (HFD) and Western diet (WD), were used to create MAFLD and MASH respectively. MC38 cancer cells were injected intrasplenically to create CRLM model. Single-cell RNA sequencing (scRNA-seq), RT-PCR and immunohistology were used to study hepatic immune-cell composition, phenotypes, and localization.
Results
Both diets significantly increased CRLM establishment, while only WD altered hepatic inflammation. The WD-promotes IL-10 and TGF-β1 elevation, an anti-inflammatory cytokines, inhibiting cytotoxic CD8+ T cells and NK cells and supporting an immunosuppressive environment. Although MASH led to an increased presence of hepatic CD8+ and NK cells, their infiltration into metastatic foci was reduced and was associated with a decrease in expression of cytotoxic markers. In our murine model of MASH, CD8+ T-cell depletion reduced the number of CRLM foci, which was accompanied by a decrease in IFN-γ-associated cytokines and a significant increase in the infiltration of granzyme-B expressing NK cells, ultimately enhancing cytotoxic killing ability.
Conclusions
This research underscores the crucial influence of diet-induced immune changes on CRLM establishment and progression. It illustrates that the co-localization of immune cells within liver metastases significantly affects their functionality, highlighting potential therapeutic strategies to balance immune exhaustion and activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


