AKI的多靶点肾保护:运动介导的AMPK能量稳态协调、mTOR自噬调节和NF-κB炎症控制
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:有氧运动(AE)可以预防急性肾损伤(AKI),但其机制尚未完全阐明。我们研究了AE预处理如何通过转录组重编程、炎症调节、自噬调节和代谢适应来预防败血症诱导的AKI。方法在AKI诱导前进行4周AE。我们量化了肾功能生物标志物、氧化应激标志物、细胞因子和代谢参数,进行了转录组学分析,并使用mTOR激动剂MHY1485验证了机制。结果sae预处理通过减少炎症和氧化损伤,显著提高患者存活率,减轻AKI。通过提高ATP/ADP比值、NAD+/NADH比值和磷酸肌酸水平,减少乳酸积累,显著改善肾功能指标(血尿素氮、肌酐、尿酸、肾小球滤过率)水平,改善代谢。转录组学分析显示,在lps诱导的AKI组(ALI组与Con组)中,有3595个差异表达基因(deg)在AMPK、mTORC1、NF-κB和TNF通路中富集。然而,AE预处理诱导的转录组重编程以392个DEGs为特征(ALI组与AE + ALI组),AMPK、mTORC1和NF-κB信号通路显著富集。运动通过三种协同机制改善AKI:(1) AMPK激活通过增强pgc -1α介导的线粒体生物发生和PPARα/ cpt1a驱动的脂肪酸氧化来恢复能量稳态;(2) mTORC1激活通过抑制ULK1-ATG13-FIP200复合体抑制过度自噬;(3)通过双重抑制IL-1R1/TAK1和TLR3/MyD88通路实现NF-κB抑制,减少促炎细胞因子。值得注意的是,MHY1485激活mTOR显著提高了存活率,减轻了肾损伤,促进了能量代谢,抑制了过度的自噬。结论sae通过调节ampk介导的代谢重编程、mtor依赖的自噬控制和NF-κB炎症抑制,在AKI中发挥多靶点肾保护作用。这项研究描述了运动诱导肾保护的分子基础,并确定mTOR是AKI的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
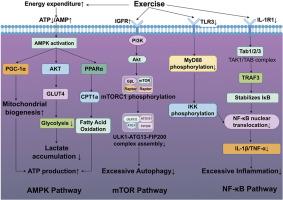
Multi-target renal protection in AKI: Exercise-mediated coordination of AMPK energy homeostasis, mTOR autophagy regulation, and NF-κB inflammatory control
Background
Aerobic exercise (AE) confers protection against acute kidney injury (AKI), but mechanisms remain incompletely elucidated. We investigated how AE preconditioning protects against sepsis-induced AKI through transcriptomic reprogramming, inflammatory regulation, autophagy modulation, and metabolic adaptation.
Methods
Mice were subjected to 4-week AE before AKI induction. We quantified renal function biomarkers, oxidative stress markers, cytokines, and metabolic parameters, performed transcriptomic analysis, and validated mechanisms using mTOR agonist MHY1485.
Results
AE preconditioning significantly increased survival rates and attenuated AKI by reducing inflammatory and oxidative damage. It significantly improved the renal dysfunction marker (blood urea nitrogen, creatinine, uric acid, and glomerular filtration rate) levels and improved metabolism by increasing the ATP/ADP ratio, NAD+/NADH ratio, and phosphocreatine level and decreasing lactate accumulation. Transcriptomic profiling revealed substantial gene expression alterations in the LPS-induced AKI group (ALI vs. Con groups), with 3595 differentially expressed genes (DEGs) that were enriched in AMPK, mTORC1, NF-κB, and TNF pathways. However, AE preconditioning induced transcriptomic reprogramming characterized by 392 DEGs (ALI vs. AE + ALI groups), which were significantly enriched in AMPK, mTORC1 and NF-κB signaling pathways. Exercise ameliorated AKI through three synergistic mechanisms: (1) AMPK activation restored energy homeostasis by enhancing PGC-1α-mediated mitochondrial biogenesis and PPARα/CPT1a-driven fatty acid oxidation; (2) mTORC1 activation suppressed excessive autophagy via ULK1-ATG13-FIP200 complex inhibition; and (3) NF-κB inhibition was achieved through dual suppression of IL-1R1/TAK1 and TLR3/MyD88 pathways, reducing pro-inflammatory cytokines. Notably, mTOR activation by MHY1485 markedly increased survival rates, attenuated renal injury, promoted energy metabolism, and suppressed excessive autophagy.
Conclusions
AE exerts multi-target nephroprotection in AKI by regulating AMPK-mediated metabolic reprogramming, mTOR-dependent autophagy control, and NF-κB inflammatory suppression. This study delineate the molecular basis of exercise-induced renal protection and identifies mTOR as a potential therapeutic target for AKI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


