m6A修饰介导的ETS2翻译上调驱动砷诱导的精原细胞衰老
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
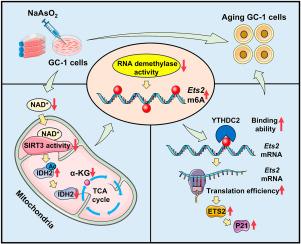
越来越多的证据表明,砷(As)暴露会导致精子质量下降。本研究旨在探讨砷暴露对精原细胞衰老的影响。GC-1细胞暴露于NaAsO2 (10 μM)中。RNA测序和核糖体分析测序鉴定细胞衰老的关键调控因子。甲基化RNA免疫沉淀- qpcr检测n6 -甲基腺苷(m6A)修饰。结果表明,这些差异表达基因在细胞衰老相关通路中富集。在砷暴露的GC-1细胞中,几种已建立的衰老标志物,β-半乳糖苷酶活性,γ-H2AX和P16均升高。进一步分析发现P21及其转录因子ETS2表达上调。敲除ETS2可阻止as诱导的P21上调和细胞衰老。多组学联合分析表明,As暴露通过ythdc2依赖性m6A修饰提高了ETS2的翻译效率。机制上,砷暴露诱导线粒体功能障碍。α-酮戊二酸(α-KG),一种三羧酸循环中间体和RNA去甲基化酶辅助因子,在砷暴露的GC-1细胞中减少。其他实验表明,砷暴露诱导NAD+消耗并抑制SIRT3活性。补充烟酰胺单核苷酸(NAD+前体)可减弱as诱导的α-KG还原和ETS2上调。这些发现表明,As通过m6A修饰介导的ETS2翻译上调诱导精原细胞衰老,并确定NAD+补充是潜在的对策。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The m6A modification-mediated upregulation of ETS2 translation drives arsenic-induced spermatogonial senescence
Accumulating evidence indicates that arsenic (As) exposure causes a decline in sperm quality. This study aimed to investigate the impact of As exposure on spermatogonial senescence. GC-1 cells were exposed to NaAsO2 (10 μM). RNA sequencing and ribosome profiling sequencing were performed to identify key regulators of cellular senescence. Methylated RNA immunoprecipitation-qPCR was used to determine N6-methyladenosine (m6A) modification. The results revealed that the differentially expressed genes were enriched in pathways related to cellular senescence. Several established senescence markers, β-galactosidase activity, γ-H2AX, and P16, were elevated in As-exposed GC-1 cells. Further analysis revealed that P21 and its transcription factor ETS2 were upregulated. ETS2 knockout prevented As-induced P21 upregulation and cell senescence. The multi-omics joint analysis indicated that As exposure elevated ETS2 translation efficiency by YTHDC2-dependent m6A modification. Mechanistically, As exposure induced mitochondrial dysfunction. Alpha-ketoglutarate (α-KG), a tricarboxylic acid cycle intermediate and RNA demethylase cofactor, was reduced in As-exposed GC-1 cells. Additional experiments showed that As exposure induced NAD+ depletion and suppressed SIRT3 activity. Supplementation with nicotinamide mononucleotide, an NAD+ precursor, attenuated As-evoked α-KG reduction and ETS2 upregulation. These findings suggest that As induces spermatogonial senescence via m6A modification-mediated upregulation of ETS2 translation and identify NAD+ replenishment as a potential countermeasure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


