运动训练通过下调STING来改善高脂饮食诱导的骨骼肌萎缩和铁下垂
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:高脂肪饮食(HFD)诱导的肌肉减少性肥胖可导致肌纤维直径减少、蛋白质降解增强和各种形式的细胞死亡。运动训练已被证明可以减轻足癣引起的肌肉萎缩。然而,其潜在机制尚不清楚。干扰素基因刺激因子(STING)参与了铁下垂和各种形式的肌肉萎缩。本研究旨在探讨STING在运动训练中对抗hfd诱导的骨骼肌萎缩的作用。方法在体内,hdd喂养小鼠进行运动训练,并腹腔注射STING激动剂diABZI或选择性STING抑制剂C-176 8周。在体外,分化的C2C12肌管先用棕榈酸(PA)处理,然后用他汀铁素-1 (fer1)、Erastin、diABZI或C-176干预。握力测试、体成分分析、血清分析、组织学分析、双氢乙酯染色、透射电镜、肌球蛋白重链染色、线粒体膜电位、Western blot、实时定量PCR。结果在体内,运动训练显著降低了STING mRNA和蛋白的表达,改善了hfd喂养小鼠骨骼肌萎缩和脂质过氧化相关的铁下垂。STING激动剂diABZI减弱了运动训练对hfd诱导的骨骼肌萎缩和铁下垂的缓解作用。选择性STING抑制剂C-176和运动训练协同缓解hfd诱导的骨骼肌萎缩和铁下垂。在体外,铁下垂抑制剂Fer-1部分挽救了pa触发的C2C12肌管萎缩和铁下垂,而铁下垂激活剂Erastin则加重了肌管萎缩和铁下垂。diABZI加重了pa诱导的C2C12肌管萎缩和铁下垂。Erastin破坏了C-176对pa诱导的C2C12肌管萎缩和铁下垂的改善作用。结论运动训练可有效抑制hfd介导的骨骼肌STING上调。STING是运动训练对hfd诱导的骨骼肌萎缩和铁下垂缓解作用的反应因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
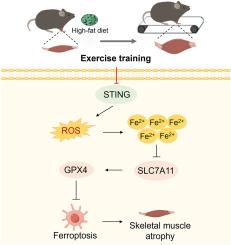
Exercise training ameliorates high-fat diet-induced skeletal muscle atrophy and ferroptosis via downregulation of STING
Background
High-fat diet (HFD)-induced sarcopenic obesity can lead to reductions in muscle fiber diameter, enhanced protein degradation, and various forms of cell death. Exercise training has been shown to alleviate HFD-induced muscle atrophy. However, the underlying mechanism remains unclear. Stimulator of interferon genes (STING) is involved in ferroptosis and various forms of muscle atrophy. This study aimed to investigate the role of STING in exercise training against HFD-induced skeletal muscle atrophy.
Methods
In vivo, HFD-fed mice were subjected to exercise training and were intraperitoneally injected with the STING agonist diABZI or selective STING inhibitor C-176 for 8 weeks. In vitro, the differentiated C2C12 myotubes were treated with palmitic acid (PA), followed by interventions with Ferrostatin-1 (Fer-1), Erastin, diABZI or C-176. Grip strength test, body composition analysis, serum assay, histology analysis, dihydroethidium staining, transmission electron microscopy, myosin heavy chain staining, mitochondrial membrane potential, Western blot, and real-time quantitative PCR were performed.
Results
In vivo, exercise training significantly reduced the mRNA and protein expression of STING and ameliorated skeletal muscle atrophy and lipid peroxidation associated ferroptosis in HFD-fed mice. The STING agonist diABZI blunted the alleviative effect of exercise training in HFD-induced skeletal muscle atrophy and ferroptosis. The selective STING inhibitor C-176 and exercise training synergistically alleviated HFD-induced skeletal muscle atrophy and ferroptosis. In vitro, the ferroptosis inhibitor Fer-1 partially rescued PA-triggered C2C12 myotubes atrophy and ferroptosis, whereas the ferroptosis activator Erastin aggravated myotubes atrophy and ferroptosis. diABZI exacerbated PA-induced C2C12 myotubes atrophy and ferroptosis. Erastin impaired the ameliorative effect of C-176 in PA-induced C2C12 myotubes atrophy and ferroptosis.
Conclusions
Exercise training effectively suppressed HFD-mediated upregulation of STING in skeletal muscle. STING is a response factor for the alleviative effect of exercise training in HFD-induced skeletal muscle atrophy and ferroptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


