枯草芽孢杆菌的铜原血红素脱羧酶是细菌生长和厌氧条件下血红素b生物合成所必需的
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
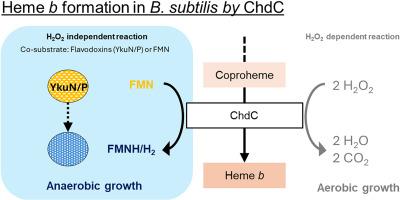
血红素的生物合成并不遵循一个普遍的途径。相反,不同的生物体利用不同的途径来产生这种基本分子。coproporphyrin-dependent (CPD)途径是革兰氏阳性细菌所特有的。鉴于革兰氏阳性病原菌的普遍存在,深入研究其酶促步骤是开发新型抗生素的先决条件。这里的重点是铜原血红素脱羧酶(ChdC,原HemQ),该途径的末端步骤,催化Fe-coproporphyrin III(铜原血红素)通过氧化脱羧转化为血红素b。在以前的研究中,过氧化氢(H2O2)已被证明是ChdC的必要的共底物和电子受体。然而,由于其细胞毒性作用,H2O2需要严格的细胞内控制,在体内,特别是在厌氧生长过程中,是一个次优底物。为了研究无h2o2的血红素生物合成途径,对革兰氏阳性模式生物枯草芽孢杆菌进行了敲除研究。这些表明ΔchdC菌株在好氧和厌氧生长过程中表现出血红素营养不良行为,突出表明ChdC可能没有厌氧替代品。游离和蛋白结合的FMN(以枯草芽孢杆菌黄毒毒素YkuN和YkuP的形式)随后被表征为可选的共底物。它们与枯草芽孢杆菌异源表达ChdC的反应在不同环境下进行了表征。通过极谱双氧水平测定、液相色谱、质谱和时间分辨光谱,这些反应被证明是可能的,并在厌氧条件下和高ph值下被促进。总之,本研究的结果证实了ChdC在革兰氏阳性模式生物中进行厌氧反应的必要性和能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coproheme decarboxylase from Bacillus subtilis is required for bacterial growth and heme b biosynthesis under anaerobic conditions
Heme biosynthesis notably does not follow a universal pathway. Instead, different organisms utilize various routes of producing this essential molecule. The coproporphyrin-dependent (CPD) pathway is unique to Gram-positive bacteria. Given the ubiquity of Gram-positive pathogenic organisms, thorough research on its enzymatic steps is a prerequisite for the development of novel antibiotics. Here the focus lies on coproheme decarboxylase (ChdC, formerly HemQ), the terminal step of the pathway, catalyzing the transformation of Fe-coproporphyrin III (coproheme) to heme b by oxidative decarboxylation. In previous studies, hydrogen peroxide (H2O2) has been shown to act as a necessary co-substrate and electron acceptor for ChdC. However, H2O2, due to its cytotoxic effects, needs tight intracellular control and is a sub-optimal substrate in vivo, especially during anaerobic growth. To investigate a H2O2-free pathway for heme biosynthesis, knockout studies on Gram-positive model organism Bacillus subtilis have been performed. These reveal that ΔchdC strains exhibit heme auxotrophic behavior during aerobic and anaerobic growth, highlighting that ChdC has likely no anaerobic alternative. Free and protein bound FMN (in the form of flavodoxins YkuN and YkuP from B. subtilis) were subsequently characterized as alternative co-substrates. Their reaction with heterologously expressed ChdC from B. subtilis was characterized in different settings. By polarographic dioxygen level determination, liquid chromatography, mass spectrometry and time-resolved spectroscopy, these reactions were shown to be possible and promoted under anaerobic conditions and at elevated pH-values. Overall, the results presented in this study confirm the necessity and the capability of ChdC to react anaerobically in a Gram-positive model organism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


