通过双机制修饰的新型仿血小板生成素肽的设计与合成
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
血小板生成素(TPO)模拟肽激活c-Mpl受体介导的信号传导来刺激血小板产生,具有与内源性TPO相似的生物活性。然而,它们的临床应用受到体内快速清除(t₁/ 2≈1 h)的限制。为了解决这一限制,各种长效修饰策略,如聚乙二醇化和Fc融合,已经被广泛开发。在这项研究中,我们通过整合两种长效技术设计了一种新的分子,它们具有互补的机制:(1)脂肪酸缀合增强白蛋白结合,(2)XTENylation增加流体动力半径。该新型TPO模拟肽具有与romiplostim相当的体外活性(0.12±0.076 nM比romiplostim 0.10±0.026 nM),在正常小鼠中具有与romiplostim相当的体内血小板升高效果,同时具有卓越的药代动力学特性:消除半衰期延长(10.86±0.36 h比对照组2.93±0.05 h),最大血药浓度升高(184.57±64.00 ng/mL比对照组93.22±60.99 ng/mL)。这些发现验证了双机制修饰的协同效应,并为开发具有可调药代动力学特征的下一代TPO疗法提供了新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
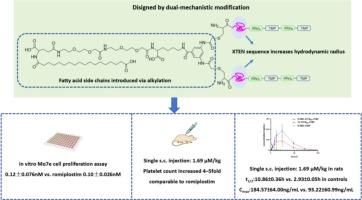
Design and synthesis of novel thrombopoietin mimetic peptides via dual-mechanistic modification
Thrombopoietin (TPO) mimetic peptides activate c-Mpl receptor-mediated signaling to stimulate platelet production, possessing similar bioactivity to endogenous TPO. However, their clinical application is limited by rapid clearance in vivo (t₁/₂ ≈ 1 h). To address this limitation, various long-acting modification strategies, such as PEGylation and Fc fusion, have been extensively developed. In this study, we designed a new molecule by integrating two long-acting technologies with complementary mechanisms: (1) fatty acid conjugation to enhance albumin binding, and (2) XTENylation to increase hydrodynamic radius. The novel TPO mimetic peptide demonstrated comparable in vitro activity (0.12 ± 0.076 nM vs. romiplostim 0.10 ± 0.026 nM) and comparable in vivo platelets elevating efficacy in normal mice to romiplostim while exhibiting superior pharmacokinetic properties: prolonged elimination half-life (10.86 ± 0.36 h vs. 2.93 ± 0.05 h in controls) and elevated maximum plasma concentration (184.57 ± 64.00 ng/mL vs. 93.22 ± 60.99 ng/mL in controls). These findings validate the synergistic efficacy of dual-mechanistic modifications and provide a novel strategy for developing next-generation TPO therapeutics with tunable pharmacokinetic profiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


