金属-阿莫地喹配合物的合成、表征、β-血红素形成的抑制、亲脂性和抗疟活性
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
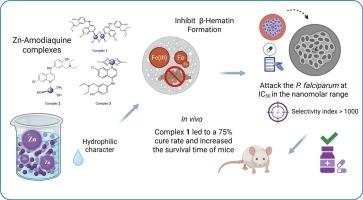
疟疾造成的死亡人数超过任何其他传染病。它每年夺去60多万人的生命。尽管正在进行努力,但根除它的有效科学努力尚未实现。根据我们寻找合理替代治疗的策略,我们开发了五种新的含amodiaquine (AQ)的配位化合物:[Zn(AQ)Cl₂]2 (1);[锌(AQ) (OH)₂₂O (H)] (2);[锌(AQ) (Ac)₂](3);在温和条件下合成的[Cu(AQ)(Ac)₂](4)和[Ag(AQ)(PPh₃)₂(H₂O)]NO₃(5)。采用电导率测量、元素和热分析、电子、红外和核磁共振光谱、ESI-MS光谱和EPR对M-AQ配合物进行了表征。AQ通过喹啉环上的氮原子与金属配位。总的来说,化合物1、2和3比单独使用阿莫地喹表现出更强的抑制效果。根据这些IC50值,我们可以推断这些金属-AQ配合物与AQ一样有效地抑制β-血红素的形成,这与它们对Fe(III)PPIX(原卟啉IX)的强结合亲和力是一致的。所有金属- aq配合物在pH为5时均呈现负对数D值,表明其亲水性更强。所有金属- aq配合物对恶性疟原虫抗cq菌株均表现出体外活性。Zn-AQ配合物对cq抗性菌株PfW2的活性最高,与AQ相当,高于二磷酸氯喹。在剂量为30 mg/kg时,复合物1治疗使小鼠的中位生存时间增加了75%。这可能是由于在其组成中存在两个阿莫地喹分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, characterization, inhibition of β-hematin formation, lipophilicity, and antimalarial activity of metal-amodiaquine complexes
Malaria kills more people than any other infectious disease. It claims over 600,000 lives each year. Despite ongoing efforts, an effective scientific endeavor to eradicate it has yet to be realized. In line with our strategy of searching for a rational alternative treatment, we have developed five new coordination compounds containing amodiaquine (AQ): [Zn(AQ)Cl₂]₂ (1); [Zn(AQ)(OH)₂(H₂O)] (2); [Zn(AQ)(Ac)₂] (3); [Cu(AQ)(Ac)₂] (4) and [Ag(AQ)(PPh₃)₂(H₂O)]NO₃ (5) which were synthesized under mild conditions. The M-AQ complexes were characterized using a combination of conductivity measurements, elemental and thermal analyses, electronic, infrared, and NMR spectroscopies, ESI–MS spectrometry and EPR. AQ is coordinated to the metal by the nitrogen atom of the quinoline ring. Overall, compounds 1, 2, and 3 demonstrated greater inhibitory efficacy compared to amodiaquine alone. Based on these IC50 values, we can infer that these metal-AQ complexes are as potent in inhibiting β-hematin formation as AQ, consistent with their strong binding affinity to Fe(III)PPIX (protoporphyrin IX). All metal-AQ complexes exhibited negative log D values at pH 5 indicating a more hydrophilic character. All the Metal-AQ complexes exhibited in vitro activity against CQ-resistant strains of P. falciparum. The Zn-AQ complexes were the most active in the series, comparable to AQ and more active than chloroquine diphosphate against the CQ-resistant strain PfW2. At a dose of 30 mg/kg, treatment with complex 1 increased the median survival time of the mice by 75 %. This may be due to the presence of two amodiaquine molecules in its composition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


