受损的xct介导的胱氨酸摄取驱动丝氨酸和脯氨酸代谢重编程和骨骼肌细胞线粒体裂变
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肌肉卫星细胞(MuSC)的增殖受到氧化还原稳态和营养供应的严格调控,而这些在肌肉病变中经常被破坏。除了维持细胞氧化还原稳态的作用外,本研究还发现了胱氨酸/谷氨酸反转运蛋白xCT在增殖musc中的关键代谢作用。我们研究了从携带编码xCT的SLC7A11基因突变的小鼠分离的Slc7a11sut/sut musc中受损的xCT介导的胱氨酸进口的影响。我们使用互补的方法来研究胱氨酸进口中断如何影响谷胱甘肽(GSH)氧化还原、细胞生物能量学、线粒体动力学和代谢。Slc7a11sut/sut musc的耗氧率较低,表明线粒体氧化能力受损。这伴随着与OPA1切割和氧化还原敏感的DRP1寡聚化相关的碎片化线粒体网络。代谢组学分析显示,Slc7a11sut/sut MuSCs具有明显的代谢特征,表现为BCAAs、嘧啶、半胱氨酸、蛋氨酸和谷胱甘肽的主要差异。尽管总体生物能量通量较低,但稳定同位素示踪分析(SITA)显示,xCT缺乏增加了葡萄糖摄取,通过转硫途径将葡萄糖衍生的碳引导到新的丝氨酸生物合成中,从而为半胱氨酸生产提供燃料,部分补偿了GSH氧化还原的中断。此外,xCT缺乏引发P5CS介导的脯氨酸还原生物合成上调。通过引导谷氨酸合成脯氨酸,musc明显下调氧化磷酸化(OXPHOS)并调节细胞内谷氨酸水平,以响应胱氨酸/谷氨酸反转运蛋白功能受损。我们的研究结果强调了xCT在调节氧化还原平衡和增殖musc代谢重编程中的作用,为肌肉和氧化还原相关病理的治疗策略提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
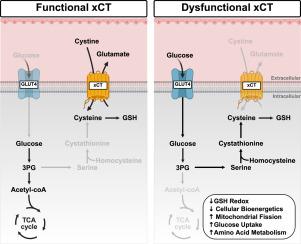
Impaired xCT-mediated cystine uptake drives serine and proline metabolic reprogramming and mitochondrial fission in skeletal muscle cells
Muscle satellite cell (MuSC) proliferation is tightly regulated by redox homeostasis and nutrient availability, which are often disrupted in muscular pathologies. Beyond its role in maintaining cellular redox homeostasis, this study identified a key metabolic role for cystine/glutamate antiporter xCT in proliferating MuSCs. We investigated the impact of impaired xCT-mediated cystine import in Slc7a11sut/sut MuSCs isolated from mice that harbor a mutation in the SLC7A11 gene, which encodes xCT. We used complementary approaches to study how disrupted cystine import affects glutathione (GSH) redox, cellular bioenergetics, mitochondrial dynamics, and metabolism. Oxygen consumption rates of Slc7a11sut/sut MuSCs were lower, indicative of compromised mitochondrial oxidative capacity. This was accompanied by a fragmented mitochondrial network associated with OPA1 cleavage and redox-sensitive DRP1 oligomerization. Metabolomic profiling revealed a distinct metabolic signature in Slc7a11sut/sut MuSCs, manifested by major differences in BCAAs, pyrimidines, cysteine, methionine, and GSH. Despite lower overall bioenergetic flux, stable-isotope tracing analyses (SITA) showed that xCT deficiency increased glucose uptake, channeling glucose-derived carbons into de novo serine biosynthesis to fuel cysteine production via the transsulfuration pathway, partially compensating for disrupted GSH redox. Furthermore, xCT deficiency triggered upregulated pyrroline-5-carboxylate synthase (P5CS)-mediated proline reductive biosynthesis. By directing glutamate into proline synthesis, MuSCs apparently downregulate oxidative phosphorylation (OXPHOS) and regulate intracellular glutamate levels in response to impaired cystine/glutamate antiporter function. Our findings highlight the roles of xCT in regulating redox balance and metabolic reprogramming in proliferating MuSCs, providing insights that may inform therapeutic strategies for muscular and redox-related pathologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


