EDTMP逆转革兰氏阴性菌金属β-内酰胺酶介导的耐药性的研究
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
金属β-内酰胺酶(MBLs)是革兰氏阴性菌对碳青霉烯耐药的主要原因。本研究开发了一种简单实用的方法合成荧光探针CE-HF,该探针可有效检测MBL NDM-1的活性。使用该探针,乙二胺四(亚甲基膦酸)(EDTMP)被鉴定为一种有效的MBL抑制剂,具有很强的活性(IC₅0 = 0.68 μM,对NDM-1)。抑菌试验(包括抑菌区、MIC和MBC试验)显示,EDTMP通过降低美罗培南和其他β-内酰胺类抗生素的MIC值,并表现出协同效应,显著增强了美罗培南和其他β-内酰胺类抗生素的疗效。棋盘实验进一步证实,EDTMP在8 μg/mL浓度下可使美罗苯南的MIC降低256倍,而锌离子拯救实验证实EDTMP通过螯合MBLs活性位点的锌离子发挥其抑制作用。此外,EDTMP恢复了美罗培南对表达L1、ImiS、IMP-1和VIM-2的重组大肠杆菌菌株以及产生VIM-2的嗜麦芽窄养单胞菌临床分离株的抗菌活性。这些发现突出了EDTMP作为广谱MBL抑制剂的作用,并提供了对抗β-内酰胺酶介导的抗生素耐药性的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
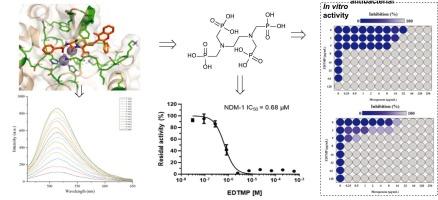
Study of the reversal of metallo-β-lactamase-mediated resistance in Gram-negative bacteria by EDTMP
Metallo-β-lactamases (MBLs) are major causes of carbapenem resistance in Gram-negative bacteria. In this study, a simple and practical method was developed to synthesize the fluorescent probe CE-HF, which was proven effective for detecting the activity of the MBL NDM-1. Using this probe, ethylenediamine tetra(methylene phosphonic acid) (EDTMP) was identified as a potent MBL inhibitor with strong activity (IC₅₀ = 0.68 μM against NDM-1). Antibacterial assays, including inhibition zone, MIC, and MBC tests, revealed that EDTMP significantly enhanced the efficacy of meropenem and other β-lactam antibiotics by lowering their MIC values and exhibiting synergistic effects. Checkerboard assays further demonstrated that EDTMP reduced the MIC of meropenem by 256-fold at 8 μg/mL, whereas zinc ion rescue experiments confirmed that EDTMP exerts its inhibitory effect by chelating zinc ions at the active site of MBLs. Moreover, EDTMP restored the antibacterial activity of meropenem against recombinant E. coli strains expressing L1, ImiS, IMP-1, and VIM-2, as well as clinical isolates of Stenotrophomonas maltophilia producing VIM-2. These findings highlight EDTMP as a broad-spectrum MBL inhibitor and offer a new strategy to combat β-lactamase-mediated antibiotic resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


