探讨低温大气等离子体诱导糖化牛血清白蛋白自组装的影响
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
牛血清白蛋白(Bovine Serum Albumin, BSA)是一种球状水溶性蛋白,由于其稳定性、结合能力和结构与人血清白蛋白(human Serum Albumin, HSA)相似,被广泛用作模型系统。冷大气等离子体(CAP)已成为生物分子修饰,灭菌,食品保存和伤口愈合的多功能工具。本研究探讨了CAP对糖基化BSA的影响,重点是结构和自组装过程。扫描电镜分析显示,CAP诱导不同的蛋白质自组装取决于处理时间。硫黄素实验显示,与天然和糖化的牛血清白蛋白相比,cap处理的糖化牛血清白蛋白荧光强度增加,表明β-片含量和自组装增强。这些发现为CAP在调节蛋白质结构中的作用提供了有价值的见解,对生物材料、疾病机制和蛋白质工程具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
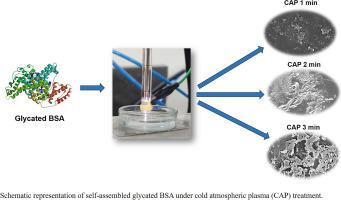
Exploring impact of cold atmospheric plasma directed self-assembly of glycated bovine serum albumin
Bovine Serum Albumin (BSA) is a globular, water-soluble protein widely used as a model system due to its stability, binding capacity, and structural similarity to human serum albumin (HSA). Cold atmospheric plasma (CAP) has emerged as a versatile tool for biomolecule modification, sterilization, food preservation, and wound healing. This study explores the effects of CAP on glycated BSA, focusing on structural and self-assembly processes. SEM analysis reveals that CAP induces distinct protein self-assemblies depending on treatment duration. Thioflavin assays show increased fluorescence intensity in CAP-treated glycated BSA compared to native and glycated BSA, indicating an enhancement in β-sheet content and self-assembly. These findings offer valuable insights into CAP's role in modulating protein structures, with implications for biomaterials, disease mechanisms, and protein engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


