牺牲氧化还原调节由分泌细菌效应分子减轻氧化应激和炎症在体内
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人与细菌的相互作用在生活的几个基本方面起着至关重要的作用。在这里,我们描述了一种来自皮肤共生痤疮角质杆菌(Cutibacterium acnes)的分泌蛋白RoxP如何作为一种牺牲性氧化还原效应分子,通过对抗氧化应激和减少应激诱导的炎症,促进与人类宿主的有益相互作用。结合结构定位、生物物理结合研究和体内实验,我们证明了RoxP如何通过作为氧化攻击的靶标和影响细胞因子信号传导来促进皮肤稳态。据我们所知,这是第一次对分泌的原核效应分子通过牺牲氧化调节宿主氧化还原平衡的体内机制描述,为其作为健康促进因子的作用提供了证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
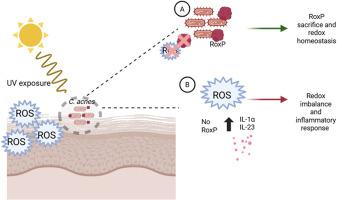
Sacrificial redox modulation by a secreted bacterial effector molecule mitigates oxidative stress and inflammation in vivo
Human-bacterial interactions play a crucial role in several essential aspects of life. Here, we describe how a secreted protein from the skin commensal Cutibacterium acnes, RoxP, functions as a sacrificial redox effector molecule that facilitates beneficial interactions with its human host by counteracting oxidative stress and reducing stress-induced inflammation. Using a combination of structural mapping, biophysical binding studies, and in vivo experiments, we demonstrate how RoxP contributes to skin homeostasis by serving as a target for oxidative attack and influencing cytokine signaling. To our knowledge, this is the first in vivo mechanistic description of a secreted prokaryotic effector molecule modulating host redox balance through sacrificial oxidation, providing evidence of its role as a health-promoting factor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


