超越光毒性:新亚甲蓝对线粒体和细胞生物能量学的阴暗面
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
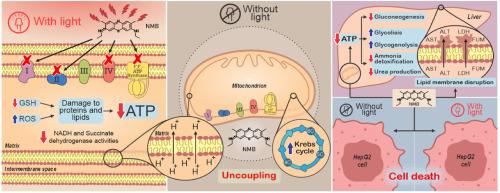
新亚甲基蓝(NMB)是一种具有公认光动力学性质的吩噻嗪染料。本研究通过三种模型(分离的大鼠肝脏线粒体、体外灌注的大鼠肝脏和HepG2细胞)评估了NMB对线粒体功能、肝脏代谢和细胞活力的光依赖性和内在(非光依赖性)影响。在离体线粒体中,即使没有照射,NMB也能解耦氧化磷酸化,降低呼吸控制(RC)和ADP/O比率。它增加了内膜通透性,从外源性NADH的氧化增强推断,并抑制线粒体肿胀。红光照射加重了这些作用,导致呼吸链复合物和FoF1-ATP合成酶的抑制,过氧化氢酶和谷胱甘肽还原酶的活性降低,以及GSH的消耗。脂质过氧化和蛋白质羰基化升高证实了氧化损伤。在灌注的肝脏中,NMB破坏了与ATP合成相关的氧气消耗,并损害了能量依赖性过程,如糖异生和氨解毒,特别是在禁食情况下。这些影响与ATP/ADP和ATP/AMP比值的降低相关,可能会损害丙酮酸羧化酶和磷酸氨基甲酰合成酶i。在喂食大鼠的肝脏中,NMB刺激糖酵解和糖原酵解,而在禁食条件下,它也增强果糖酵解——可能是对线粒体功能障碍的代偿反应。此外,nmb介导的NADH氧化可能提高NAD+的可用性并支持柠檬酸循环活性。即使没有光,也会发生膜不稳定和酶渗漏,表明具有很强的内在细胞毒性。红光照射进一步加剧了肝损伤,可能是由于光敏剂的原位激活。在HepG2细胞中,NMB以浓度依赖的方式降低细胞活力,在辐照和未辐照条件下无显著差异,突出了明显的暗毒性。这些结果表明,NMB通过光动力和非光动力机制损害线粒体功能和肝脏代谢。考虑到所有模型的一致毒性,包括在没有光的情况下,NMB作为光敏剂的治疗潜力似乎有限,在临床应用前需要仔细的毒理学评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Beyond phototoxicity: The dark side of new methylene blue on mitochondrial and cellular bioenergetics
New methylene blue (NMB) is a phenothiazine dye with recognized photodynamic properties. This study evaluated both light-dependent and intrinsic (light-independent) effects of NMB on mitochondrial function, hepatic metabolism, and cell viability using three models: isolated rat liver mitochondria, ex vivo perfused rat liver, and HepG2 cells. In isolated mitochondria, NMB uncoupled oxidative phosphorylation, decreasing the respiratory control (RC) and ADP/O ratios, even without irradiation. It increased inner membrane permeability, inferred from enhanced oxidation of exogenous NADH, and inhibited mitochondrial swelling. Red-light irradiation exacerbated these effects, leading to inhibition of respiratory chain complexes and FoF1-ATP synthase, along with reduced catalase and glutathione reductase activities and depletion of GSH. Oxidative damage was confirmed by elevated lipid peroxidation and protein carbonylation. In perfused liver, NMB disrupted oxygen consumption linked to ATP synthesis and impaired energy-dependent processes such as gluconeogenesis and ammonia detoxification, particularly under fasting. These effects correlated with decreased ATP/ADP and ATP/AMP ratios, potentially impairing pyruvate carboxylase and carbamoyl phosphate synthetase I. In livers from fed rats, NMB stimulated glycolysis and glycogenolysis, while in fasting conditions it also enhanced fructolysis—likely as a compensatory response to mitochondrial dysfunction. Additionally, NMB-mediated NADH oxidation may raise NAD+ availability and support citric acid cycle activity. Membrane destabilization and enzyme leakage occurred even without light, indicating strong intrinsic cytotoxicity. Red-light exposure further intensified hepatic damage, likely due to in situ activation of the photosensitizer. In HepG2 cells, NMB reduced cell viability in a concentration-dependent manner, with no significant difference between irradiated and non-irradiated conditions—highlighting a pronounced dark toxicity. These results demonstrate that NMB impairs mitochondrial function and hepatic metabolism through both photodynamic and non-photodynamic mechanisms. Given the consistent toxicity across all models, including in the absence of light, NMB's therapeutic potential as a photosensitizer appears limited, warranting careful toxicological assessment before clinical application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


