三唑-苯二氮卓衍生物:一锅合成,表征,赫斯菲尔德表面分析,以及作为KIF11抑制剂抗癌潜力的计算见解
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究探讨了苯二氮卓类化合物BZD1与n -芳基- c -乙氧羰基硝基亚胺3(a-b)的[3 + 2]环加成反应。该反应在碱性介质中进行,得到了选择性提高的新型三唑-苯二氮卓衍生物4(a-b)。采用1H, 13C NMR以及x射线衍射分析进行结构表征。对两种新合成的三唑-苯二氮卓衍生物4a和4b的电子结构和反应性进行了全面的理论研究。前沿分子轨道(FMO)、静电势(ESP)、福井函数和全局描述子分析一致表明,三唑-苯二氮卓核心驱动电荷转移,而酯和三唑基是亲核和亲电攻击的主要位点。根据Hirshfeld表面分析,没有观察到明显的π -π堆叠相互作用,即使弥散力(H··H接触)占主导地位,氧和氮原子之间的弱氢键也起着关键的定向作用。药物相似性和计算机ADMET研究表明,这两种化合物均表现出高的口服生物利用度、血脑屏障穿越和良好的胃肠道吸收,所有这些都表明中枢神经系统可能被激活。对接模拟结果表明,这两种化合物在开发具有潜在抗癌作用的口服生物可利用KIF11抑制剂方面具有良好的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
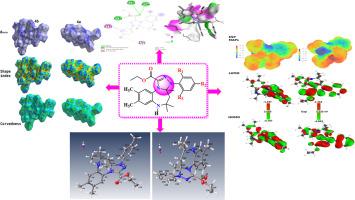
Triazole-benzodiazepine derivatives: One-pot synthesis, characterization, hirshfeld surface analysis, and computational insights into anticancer potential as KIF11 inhibitors
In this study a [3 + 2] cycloaddition reaction of the benzodiazepine BZD1 with N-aryl-C-ethoxycarbonylnitrilimines 3(a-b) was explored. The reaction was carried out in a basic medium to obtain new triazole-benzodiazepine derivatives 4(a-b) with improved selectivity. Structural characterization was performed using 1H, 13C NMR spectroscopy, as well as X-ray diffraction analysis. A comprehensive theoretical study was conducted to elucidate the electronic structure and reactivity of two newly synthesized triazole-benzodiazepine derivatives (4a and 4b). Frontier Molecular Orbital (FMO), Electrostatic Potential (ESP), Fukui function, and global descriptor analyses consistently revealed that the triazole-benzodiazepine core drives charge transfer, while the ester and triazole groups are the main sites of nucleophilic and electrophilic attacks. According to Hirshfeld surface analysis, there was no significant π–π stacking interactions were observed, and weak hydrogen bonding between oxygen and nitrogen atoms play a critical directing function, even if dispersion forces (H···H contacts) predominate. Drug similarity and in silico ADMET studies indicated that both compounds exhibit high predicted oral bioavailability, blood-brain barrier crossing, and good gastrointestinal absorption all of which suggest that the central nervous system may be activated. Docking simulation results demonstrated that both compounds represent promising prospects for the development of oral bioavailable KIF11 inhibitors with potential anticancer effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


