含大体积阴离子的十甲基和八甲基二茂铁盐的相行为:填料和阴离子结构对离子塑料晶体形成的影响
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
离子塑料晶体(IPCs)是一种由接近球形的离子在固体状态下进行旋转运动的晶体,近年来由于其独特的性能而受到人们的关注。为了研究大体积阴离子在有机金属盐中诱导IPC形成的能力,我们制备了十甲基二铁盐([1]X)和八甲基二铁盐([2]X),其中含有CB11H12 -碳硼烷阴离子或六氟丙烷-1,3-二磺酰胺阴离子(CPFSA毒血症),其中[2]CPFSA已被报道过。虽然所有盐都经历了固-固相变,但只有[1]CB11H12在494 K以上形成了IPC相。这种相行为与低温晶体结构有关;[1]CB11H12和[2]CB11H12均采用有利于IPC形成的正阴离子交替排列,但后者的局部正阴离子构型阻碍了分子的旋转。相比之下,[1]CPFSA和[2]CPFSA由于阴离子结构内的电荷局域化和阳离子配体之间的面对面相互作用诱导的离子配对,采用了非交替的正阴离子排列。因此,分子性质、局部正负离子构型和包装排列影响IPC的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
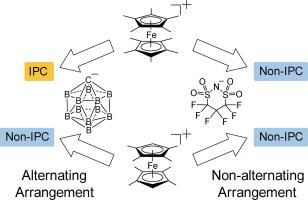
Phase behavior of decamethyl- and octamethylferrocenium salts containing bulky anions: Effects of packing and the anion structure on ionic plastic crystal formation
Ionic plastic crystals (IPCs), in which nearly spherical ions undergo rotational motion in the solid state, have recently attracted attention because of their unique properties. To investigate the ability of bulky anions to induce IPC formation in organometallic salts, we prepared decamethylferrocenium salts ([1]X) and octamethylferrocenium salts ([2]X) containing a CB11H12⁻ carborane anion or a hexafluoropropane-1,3-disulfonamide anion (CPFSA⁻), of which [2]CPFSA was previously reported. Although all the salts underwent a solid–solid phase transition, only [1]CB11H12 formed an IPC phase above 494 K. This phase behavior correlated with the low-temperature crystal structures; [1]CB11H12 and [2]CB11H12 both adopted alternating cation–anion packing arrangements favorable for IPC formation, but the local cation–anion configuration of the latter hindered molecular rotation. In contrast, [1]CPFSA and [2]CPFSA adopted non-alternating cation–anion arrangements because of ion pairing induced by charge localization within the anion structure and face-to-face interactions between cation ligands. Thus, molecular properties, local cation–anion configurations, and packing arrangements influence IPC formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


