综合转录组测序和生化研究发现,丙烯醛通过lncRNA MIR155HG/miR-155-5p/CD200R1和Nrf2/SLC7A11/GPX4铁垂通路诱导BEAS-2B细胞和雄性大鼠肺损伤
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
丙烯醛是一种重要的高优先级有害空气污染物。本研究通过结合细胞、转录组和动物水平的研究,探讨丙烯醛诱导的铁上吊与肺损伤发病机制的关系。体内和体外模型结果显示,丙烯醛暴露后共获得18,868个差异lncrna和15,639个差异mrna。经筛选,丙烯醛损伤组的636个lncrna和214个mrna与对照组有明显差异。差异基因主要富集在铁离子结合、组织稳态等方面。其中,lncRNA MIR155HG显著响应丙烯醛的毒性作用,敲低lncRNA MIR155HG可抑制miR-155-5p表达,促进CD200R1表达,提高细胞活力。此外,它可以有效抑制丙烯醛诱导的BEAS-2B细胞和肺组织中铁凋亡途径的激活,表明lncRNA MIR155HG可能是抑制丙烯醛毒性的潜在新靶点。LncRNA-MIR155HG/miR-155-5p/CD200R1轴在肺损伤中发挥重要作用,并通过调控Nrf2/SLC7A11/GPX4通路诱导铁下沉。本研究首次发现丙烯醛暴露显著影响BEAS-2B细胞中lncRNA和mRNA的表达,并发现lncRNA MIR155HG有效调控丙烯醛诱导的铁凋亡通路,为肺损伤的治疗提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
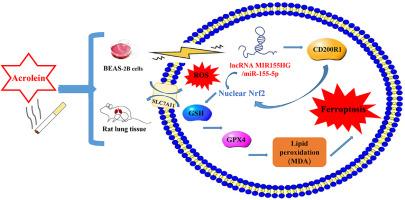
Comprehensive transcriptome sequencing and biochemical studies revealed that acrolein induced lung injury in BEAS-2B cells and male rats through the lncRNA MIR155HG/miR-155-5p/CD200R1 and Nrf2/SLC7A11/GPX4 ferroptosis pathways
Acrolein is a significant high priority hazardous air pollutant. This research explore the relationship of acrolein-induced ferroptosis with the pathogenesis of lung injury by combining cellular, transcriptome, and animal-level studies. The results in vivo and in vitro models indicated that a total of 18,868 differenced lncRNAs and 15,639 differenced mRNAs were obtained after acrolein exposure. After screening, 636 lncRNAs and 214 mRNAs were obviously different of the acrolein injured group with the control group. Differential genes were mainly enriched in the iron ion binding, tissue homeostasis, etc. Among them, lncRNA MIR155HG significantly responded to the toxic effects of acrolein, the knockdown of lncRNA MIR155HG could inhibit miR-155-5p expression, promote CD200R1 expression, and improve cell viability. Furthermore, it effectively inhibited acrolein-induced the activation of ferroptosis pathway in BEAS-2B cells and lung tissue, indicating that lncRNA MIR155HG might be a potential new target for the inhibition of acrolein toxicity. LncRNA-MIR155HG/miR-155-5p/CD200R1 axis played an significant role in the lung injury, and induced ferroptosis by regulating the Nrf2/SLC7A11/GPX4 pathway. This study is the first to reveal that acrolein exposure significantly affected lncRNA and mRNA expression in BEAS-2B cells, and found that lncRNA MIR155HG effectively regulated acrolein-induced ferroptosis pathway, providing novel insights for the treatment of lung injury.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


