n -丁苯酞(NBP)和川芎嗪(TMP)三唑复合物靶向KEAP1-NRF2通路抑制铁凋亡,发挥脑神经保护作用
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
缺血性脑卒中是一种危及生命的疾病,其病理进展涉及氧化应激、细胞凋亡、铁下垂等多种因素。我们之前的研究表明,n -丁苯酞(NBP)与川芎嗪(TMP)杂交得到了有前途的抗缺血性化合物。本研究进一步在NBP-TMP杂化物中引入三唑结构,合成了20个新化合物。在OGD/R诱导的SH-SY5Y细胞和海马原代神经元上评价其神经保护活性,在6.25 μM浓度下优选化合物8a、8b和8d,其神经保护活性优于阳性对照NBP。其中8a的保护活性最高,保护率为75.6%。进一步的机制研究表明,化合物8a、8b和8d在体外可维持细胞内氧化还原稳态,抵抗氧化应激,抑制细胞凋亡。具体而言,化合物8a通过调节KEAP1-NRF2通路发挥神经保护作用:与KEAP1结合,增强NRF2解离和核易位,促进下游抗氧化因子的产生,从而降低细胞内活性氧(ROS)水平,有效保护神经元线粒体。最后,体内实验表明,化合物8a (20 mg/kg)可显著改善缺血再灌注损伤大鼠的脑损伤。通过调节KEAP1-NRF2通路降低脑氧化应激,抑制脑内神经元凋亡和铁凋亡,与体外实验结果一致。总之,我们的研究结果表明,8a是一种很有希望的脑卒中治疗候选者,并可能促进未来抗缺血性药物的开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
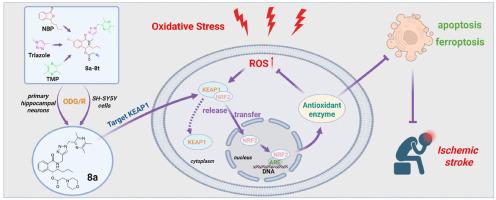
N-butylphthalide (NBP) and ligustrazine (TMP) triazole hybrids target the KEAP1-NRF2 pathway to inhibit ferroptosis and exert brain neuroprotectivity
Ischemic stroke is a life-threatening disease, its pathological progression involves multiple factors, including oxidative stress, apoptosis, and ferroptosis. Our previous study demonstrated that hybridizing N-butylphthalide (NBP) with ligustrazine (TMP) yielded promising anti-ischemic compounds. In this study, we further introduced a triazole structure into NBP-TMP hybrids and synthesized 20 novel compounds. Their neuroprotective activities were evaluated on OGD/R induced SH-SY5Y cells and primary hippocampal neurons, leading to the identification of preferred compounds 8a, 8b and 8d at the concentration of 6.25 μM, which surpassed the neuroprotective activity of the positive control NBP. Among them, 8a exhibited the highest protective activity, with a protection percentage of 75.6 %. Further mechanistic studies revealed that compounds 8a, 8b and 8d maintained intracellular redox homeostasis to resist oxidative stress and inhibit apoptosis in vitro. Specifically, compound 8a exerted neuroprotective effects by modulating the KEAP1-NRF2 pathway: it bound to KEAP1, enhanced NRF2 dissociation and nuclear translocation, facilitated the generation of downstream antioxidant factors, thereby reducing intracellular reactive oxygen species (ROS) levels and effectively protecting neuronal mitochondria. Finally, in vivo experiments demonstrated that compound 8a (20 mg/kg) significantly ameliorated cerebral injury in rats with ischemia-reperfusion injury. Furthermore, it reduced cerebral oxidative stress by modulating the KEAP1-NRF2 pathway and inhibited neuronal apoptosis and ferroptosis in the brain, which is consistent with the results in vitro. In conclusion, our results indicated that 8a serves as a promising candidate for stroke treatment and may facilitate the development of future anti-ischemic drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


