自组装双靶点布洛芬-伊立替康偶联物用于结直肠癌治疗
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
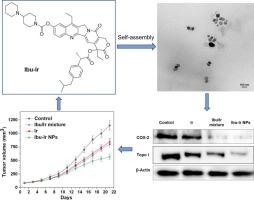
自组装纳米药物缀合物利用亲水性和疏水性配体靶向肿瘤递送,减少全身毒性,同时提高治疗效果。本文通过在疏水性非甾体抗炎药(NSAID)与亲水性Ir之间形成酯键,合成了四种新型非甾体抗炎药(NSAID)-伊立替康(irinotecan, Ir)缀合物。这些两亲性共轭物在水溶液中自组装成无载体的NSAID-Ir纳米颗粒(NPs)。其中,Ibu-Ir NPs对HT-29细胞的IC50为1.15 μM,效价为游离Ir的5.6倍。从机制上讲,Ibu-Ir NPs下调环氧化酶-2和拓扑异构酶I的表达,增强Ir的治疗效果。在HT-29异种移植模型中,Ibu-Ir NPs能够抑制肿瘤生长,尽管小样本量(n = 3)具有较高的统计误差风险。这种简单而有效的策略结合了抗炎和抗癌作用,显示出结直肠癌治疗的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-assembled dual-target ibuprofen-irinotecan conjugate for colorectal cancer therapy
Self-assembled nanodrug conjugates utilize both hydrophilic and hydrophobic ligands for targeted tumor delivery, reducing systemic toxicity while enhancing therapeutic efficacy. Herein, four novel nonsteroidal anti-inflammatory drug (NSAID)-irinotecan (Ir) conjugates were synthesized by forming ester bonds between hydrophobic NSAIDs and hydrophilic Ir. These amphiphilic conjugates self-assembled into carrier-free NSAID-Ir nanoparticles (NPs) in aqueous solution. Among them, Ibu-Ir NPs demonstrated superior activity against HT-29 cells (IC50 = 1.15 μM), showing 5.6-fold greater potency than free Ir. Mechanistically, Ibu-Ir NPs downregulated cyclooxygenase-2 and topoisomerase I expression, enhancing Ir's therapeutic effect. In HT-29 xenograft models, Ibu-Ir NPs was able to inhibit tumor growth, although the small sample size (n = 3) carries a high risk of statistical errors. This simple yet potent strategy combines anti-inflammatory and anticancer actions, demonstrating significant potential for colorectal cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


