结缔组织病相关肺动脉高压患者的非侵入性低风险标准评估:来自INSPECTIO研究的12个月分析
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
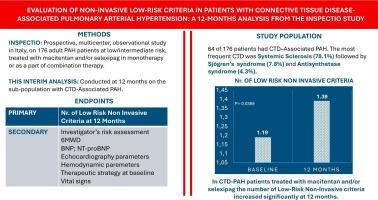
结缔组织病相关性肺动脉高压(CTD-PAH)是一种进行性、高风险的PAH亚型,其特征是免疫介导的血管重构、治疗反应差和生存率降低。这一人群的治疗反应和风险演变的真实数据仍然有限。方法:对多中心前瞻性INSPECTIO研究进行事后分析,评估了使用马西坦和/或selexipag治疗的CTD-PAH亚群。主要终点是从基线到12个月的无创低风险标准(世界卫生组织功能等级I-II, 6分钟步行距离440米,BNP 50 ng/L或NT-proBNP 300 ng/L)数量的变化。次要终点包括危险分层、6MWD、BNP/NT-proBNP水平、超声心动图和血流动力学参数的变化,并与研究中非ctd PAH人群进行比较。结果在纳入INSPECTIO研究的176例患者中,64例(36.4%)患有CTD-PAH。CTD组系统性硬化症(SSc)患者患病率较高,主要为女性(93.8%),年龄大于非CTD组(66.4岁vs 59.6岁,p = 0.0005)。从基线到第12个月,非侵入性低风险标准的平均数量显著增加(+0.20;p = 0.0389), 10.9%的CTD患者在第12个月达到三个低风险标准。次要终点(研究者风险评估、6MWD、BNP、NT-proBNP、超声心动图和血流动力学参数、基线治疗策略、生命体征)总体保持稳定。结论sctd - pah患者在马西坦和/或selexipag治疗下无创风险标准改善,功能、超声心动图和血流动力学参数稳定。尽管观察性质和小样本量,这个现实世界的分析支持使用基于风险的治疗策略和密切监测的患者群体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of non-invasive low-risk criteria in patients with connective tissue disease-associated pulmonary arterial hypertension: A 12-months analysis from the INSPECTIO study
Background
Connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) is a progressive, high-risk subtype of PAH characterized by immune-mediated vascular remodeling, poor treatment response, and reduced survival. Real-world data on therapeutic response and risk evolution in this population remain limited.
Methods
This post hoc analysis of the multicenter, prospective INSPECTIO study evaluated the CTD-PAH subpopulation treated with macitentan and/or selexipag. The primary endpoint was the change in the number of non-invasive low-risk criteria (World Health Organization functional class I–II, 6-min walk distance >440 m, BNP <50 ng/L or NT-proBNP <300 ng/L) from baseline to 12 months. Secondary endpoints included changes in risk stratification, 6MWD, BNP/NT-proBNP levels, echocardiographic and hemodynamic parameters, a comparison with the non-CTD PAH population of the study was also conducted.
Results
Among a total of 176 patients enrolled in the INSPECTIO study, 64 (36.4 %) had CTD-PAH. The CTD group, with a larger prevalence of systemic sclerosis (SSc) patients, was predominantly female (93.8 %) and older than the non-CTD group (66.4 vs. 59.6 years, p = 0.0005). The mean number of non-invasive low-risk criteria increased significantly from baseline to Month 12 (+0.20; p = 0.0389), with 10.9 % of CTD patients achieving three low-risk criteria at Month 12. Secondary endpoints (Investigator's risk assessment, 6MWD, BNP, NT-proBNP, echocardiographic and hemodynamic parameters, baseline therapeutic strategy, vital signs) remained collectively stable.
Conclusions
CTD-PAH patients showed improvement in non-invasive risk criteria and stabilization of functional, echocardiographic, and hemodynamic parameters under macitentan and/or selexipag therapy. Despite the observational nature and small sample size, this real-world analysis supports the use of risk-based treatment strategies and close monitoring in this patient population.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


