高达30兆帕的水聚乙二醇单十二烷基醚系统的溶解度行为:测量和相关
IF 2.2
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
非离子表面活性剂水溶液表现出相分离为两种不同的液体胶束相:低表面活性剂浓度的稀相和富含表面活性剂的凝聚相。这些表面活性剂在水介质中溶质萃取过程中的应用越来越多,这凸显了了解其溶解度行为的重要性。本研究报告了聚乙二醇单十二烷基醚二元水混合物系统的云点数据,其乙氧基化程度(6,7,8,9和10)不等。当温度以0.1 K/min的恒定速率变化时,在恒定压力高达30 MPa(4个等压压)下,通过监测混合物的浊度出现和消失,在高压仪器中检测云点。所研究的体系的混相行为受到乙氧基化程度和施加压力的积极影响。应用Flory-Huggins (FH)方程来关联溶解度曲线,允许在观察温度范围内生成伪实验联系线。利用非随机双液(NRTL)模型对这些联系线进行了进一步的关联,该模型对相互作用参数具有线性温度依赖性。Flory-Huggins和NRTL模型在实验不确定度范围内显示出一致性,温度的均方根偏差(RMSD)分别为0.5 K和0.1%。指出了这些模型的可行的工艺应用,包括通过化学驱提高采收率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
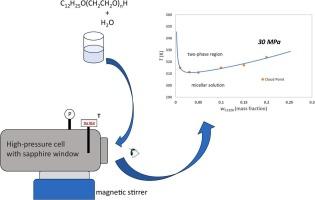
Solubility behavior for aqueous polyethylene glycol monododecyl ether systems up to 30 MPa: measurement and correlation
Nonionic surfactant aqueous solutions exhibit phase separation into two distinct liquid micellar phases: a dilute phase with a low surfactant concentration and a surfactant-rich phase, known as coacervate. The application of these surfactants in solute extraction processes from aqueous media has been increasing, highlighting the importance of understanding their solubility behavior. This work reports cloud point data of a systematic series of binary aqueous mixtures of polyethylene glycol monododecyl ethers, ranging the degree of ethoxylation (6, 7, 8, 9, and 10). The cloud points were detected in a high-pressure apparatus by monitoring the turbidity appearance and disappearance of the mixtures as the temperature changed at a constant rate of 0.1 K/min, under constant pressures up to 30 MPa (4 isobarics). The miscibility behavior of the studied systems was positively affected by both the degree of ethoxylation and the applied pressure. The Flory-Huggins (FH) equation was applied to correlate the solubility curves, allowing the generation of pseudo-experimental tie lines across the observed temperature range. These tie lines were further correlated using the nonrandom two-liquid (NRTL) model with a linear temperature dependence for the interaction parameters. The Flory-Huggins and NRTL models showed agreement within the bounds of experimental uncertainty, with root-mean-square deviations (RMSD) of 0.5 K for temperature and 0.1 % for composition, respectively. Feasible process applications of these models, including enhanced oil recovery through chemical flooding, are indicated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Thermodynamics
工程技术-热力学
CiteScore
5.60
自引率
15.40%
发文量
199
审稿时长
79 days
期刊介绍:
The Journal of Chemical Thermodynamics exists primarily for dissemination of significant new knowledge in experimental equilibrium thermodynamics and transport properties of chemical systems. The defining attributes of The Journal are the quality and relevance of the papers published.
The Journal publishes work relating to gases, liquids, solids, polymers, mixtures, solutions and interfaces. Studies on systems with variability, such as biological or bio-based materials, gas hydrates, among others, will also be considered provided these are well characterized and reproducible where possible. Experimental methods should be described in sufficient detail to allow critical assessment of the accuracy claimed.
Authors are encouraged to provide physical or chemical interpretations of the results. Articles can contain modelling sections providing representations of data or molecular insights into the properties or transformations studied. Theoretical papers on chemical thermodynamics using molecular theory or modelling are also considered.
The Journal welcomes review articles in the field of chemical thermodynamics but prospective authors should first consult one of the Editors concerning the suitability of the proposed review.
Contributions of a routine nature or reporting on uncharacterised materials are not accepted.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


