参灵白珠散通过抑制PI3K/AKT/NF-κB信号通路治疗病毒性肺炎的机制及波谱效应关系
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
参灵白珠散(SLBZS)是一种文献丰富、应用广泛的传统方剂,用于治疗病毒性肺炎。然而,SLBZS治疗病毒性肺炎的关键有效成分及其作用机制尚不清楚。本研究旨在探讨SLBZS对病毒性肺炎的作用,鉴定其生物活性成分,并揭示其可能的作用机制。材料与方法建立病毒性肺炎小鼠模型,评价SLBZS的治疗效果。随后,本研究采用UPLC-Q-TOF-MS对SLBZS在体内和体外条件下的成分进行了分析。利用网络药理学和GEO数据库预测了潜在的通路和靶点。此外,通过光谱效应关系分析确定潜在有效成分,并利用偏最小二乘回归分析(PLSR)和分子对接对其药效进行验证。结果slbzs治疗可显著减轻H1N1感染小鼠的肺部炎症损伤,抑制病毒诱导的炎症因子的产生。从生化角度看,它逆转了H1N1感染引起的肺组织中p-PI3K/T-PI3K、p-AKT/T-AKT、p-NF-κB p65/NF-κB p65的过表达。本研究共鉴定出37种体外成分和35种体内成分,其中4种成分可显著调节过度活跃免疫反应与有益炎症过程之间的平衡。此外,分子对接研究表明,白术素III和桔梗素D具有抑制PI3K-AKT-NF-κB信号通路的潜力。结论slbzs通过抑制PI3K-AKT-NF-κB信号通路的过度激活,可有效缓解h1n1诱导的病毒性肺炎。此外,本研究提出了一种创新的方法来阐明含药血清和组织的化学和药理学特征之间的相关性。通过应用这种方法,能够调节过度活跃的免疫反应和炎症之间的平衡的生物活性化合物已被成功鉴定。其中,白术内酯III和桔梗素D作为潜在的PI3K抑制剂,具有进一步探索的价值。总的来说,这些发现为开发利用SLBZS及其活性成分的新型病毒性肺炎治疗策略提供了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
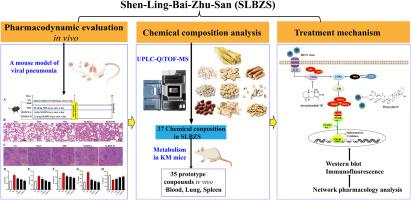
Mechanism and spectrum effect relationship of Shen-Ling-Bai-Zhu-San in treating viral pneumonia via inhibiting the PI3K/AKT/NF-κB signaling pathway
Ethnopharmacological relevance
Shen-ling-bai-zhu-san (SLBZS), a well-documented and widely used traditional formula, is used to treat viral pneumonia. However, the key active ingredients and mechanism of SLBZS in treating viral pneumonia are not clear.
Aim of the study
This study aimed to investigate the effect of SLBZS on viral pneumonia, identify its bioactive components, and reveal the possible mechanism.
Materials and methods
A mouse model with viral pneumonia was established to assess SLBZS's therapeutic impact. Subsequently, this study employed UPLC-Q-TOF-MS to analyze the components of SLBZS under both in vivo and in vitro conditions. Potential pathways and targets were predicted with the assistance of network pharmacology and the GEO database. Furthermore, potential active components were identified through spectrum-effect relationship analysis, and their efficacy was validated using Partial Least Squares Regression Analysis (PLSR) and molecular docking.
Results
SLBZS treatment significantly alleviated pulmonary inflammatory injury in mice infected with H1N1 and suppressed the production of inflammatory factors induced by the virus. Biochemically, it reversed the overexpression of p-PI3K/T-PI3K, p-AKT/T-AKT, and p-NF-κB p65/NF-κB p65 in lung tissues caused by H1N1 infection. This study identified 37 in vitro components and 35 in vivo components of SLBZS, among which four were found to significantly regulate the balance between overactive immune responses and beneficial inflammatory processes. Additionally, molecular docking studies indicated that atractylodin III and platycodin D have the potential to inhibit the PI3K-AKT-NF-κB signaling pathway.
Conclusion
SLBZS effectively alleviates H1N1-induced viral pneumonia by suppressing the overactivation of the PI3K-AKT-NF-κB signaling pathway. Furthermore, this study presents an innovative approach to clarify the correlation between the chemical and pharmacological profiles of drug-containing serum and tissues. By applying this method, bioactive compounds capable of regulating the balance between overactive immune responses and inflammation have been successfully identified. Among these, atractylenolide III and platycodin D stand out as potential PI3K inhibitors, which underscores their value for further exploration. Collectively, these findings provide a robust foundation for the development of novel therapeutic strategies for viral pneumonia, leveraging SLBZS and its active constituents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


