CO2、H2S和CH4在咪唑基离子液体中的渗透行为:分子动力学研究
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文研究了离子液体[BMIM][SCN]和[BMIM][DCA]中CO₂,H₂S和CH₄在298 K和373 K下的吸收和输运行为。自由能分布和密度分布分析表明,CO₂和H₂S优先积聚在il -真空界面,[BMIM][DCA]表现出更强的表面相互作用和更高的渗透障碍。径向分布函数分析表明,CO₂与IL阴离子形成最明显的局部关联,而CH₄在两种IL中都表现出弱的非特异性相互作用。扩散系数计算表明,气体迁移率随温度升高而增加,在较低温度下,[BMIM][SCN]中的气体迁移率始终较高,但在373 K时,[BMIM][DCA]中的气体迁移率上升得更为急剧。这些结果为控制氰化物功能化离子液体中气体选择性和输运的因素提供了全面的分子水平见解,为合理设计基于il的气体分离工艺提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
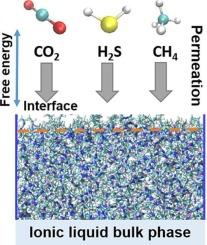
Permeation behavior of CO2, H2S, and CH4 in imidazolium-based ionic liquids: A molecular dynamics study
This work investigates the absorption and transport behavior of CO₂, H₂S, and CH₄ in the ionic liquids [BMIM][SCN] and [BMIM][DCA] using molecular dynamics simulations with umbrella sampling at 298 K and 373 K. Analysis of free energy profiles and density distributions shows that CO₂ and H₂S preferentially accumulate at the IL-vacuum interface, with [BMIM][DCA] exhibiting stronger surface interactions and higher barriers for permeation into the bulk. Radial distribution function analysis reveals that CO₂ forms the most pronounced local associations with IL anions, while CH₄ displays weak, non-specific interactions in both ILs. Diffusion coefficient calculations demonstrate that gas mobility increases with temperature and is consistently higher in [BMIM][SCN] at lower temperature, but rises more sharply in [BMIM][DCA] at 373 K. These results provide comprehensive molecular-level insights into the factors governing gas selectivity and transport in cyanide-functionalized ionic liquids, informing the rational design of IL-based gas separation processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


