暴露于三唑磷的菲律宾鲷鱼鳃的蛋白质组学分析:氧化应激,细胞骨架改变,蛋白质稳态和能量代谢增强的见解
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-08-20
DOI:10.1016/j.cbd.2025.101613
引用次数: 0
摘要
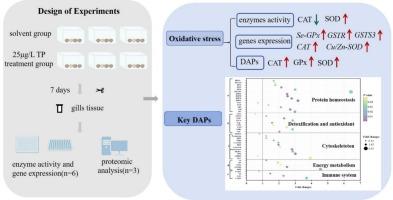
三唑磷(TP)的广泛使用对水生生物,特别是蛤类等非靶生物造成了威胁。本研究旨在评价TP亚致死浓度对菲律宾蛤蚌的影响。将TP浓度为25 μg/L(相当于96 h LC50值的2%)的条件下处理7 d,观察蛤鳃中蛋白质组学变化、抗氧化酶活性和基因表达水平。蛋白质组学结果显示,TP暴露在鱼鳃中诱导了62个差异丰度蛋白(DAPs),其中51个丰度较高,11个丰度较低。结合酶活性(含量)和基因表达分析结果,我们发现TP胁迫7 d后,参与氧化应激、细胞骨架调节、蛋白质稳态和能量代谢途径的蛋白质丰度发生了显著变化。这表明,在低浓度下,TP可能激活应激反应途径或细胞防御策略,从而导致某些蛋白质的上调。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Proteomic profiling of Ruditapes philippinarum gills exposed to triazophos: Insights into oxidative stress, cytoskeletal alterations, protein homeostasis and energy metabolism enhancement
The extensive utilization of triazophos (TP) caused a threat to aquatic organisms, particularly the non-target organisms such as clams. The current research aimed to evaluate the impact of sub-lethal concentration of TP on Malina clam Ruditapes philippinarum. Clams were subjected to a TP concentration at 25 μg/L (equivalent to 2 % of the 96 h LC50 value) for a period of 7 d, then the proteomic alterations, as well as the activities and gene expression levels of antioxidant enzymes in the clam gills were examined. Proteomics revealed that TP exposure induced 62 differentially abundant proteins (DAPs) in the gills, including 51 with higher abundance and 11 lower abundance. Combined with the findings of enzyme activities (contents) and gene expression analysis, we observed significant variations in the abundance of proteins involved in oxidative stress, cytoskeletal regulation, protein homeostasis, and energy metabolism pathways in the gills under 7 d of TP stress. This suggested that at low concentration, TP might activate stress response pathways or cellular defense strategies, thereby leading to the upregulation of certain proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


