异香豆素a - m:红树林内生真菌Alternaria sp. HN-17中具有抗炎和抗结核活性的异香豆素
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从红树林内生真菌Alternaria sp. n -17中分离得到15个新的异香豆素衍生物1-3、(±)-4、(±)-5、6-10、(+)-11、(+)-12和13,以及11个已知的类似物(−)-11、(−)-12和14-22。通过1D/2D NMR和hresms光谱以及电子圆二色性(ECD)计算,明确了它们的结构。异香豆素A(1)具有罕见的6/6/6/5四环骨架,其核心为γ-丁内酯-二苯并-α-吡酮,而2和3具有罕见的氯化呋喃环体系。在生物实验中,化合物1、(−)-4、10和18比阳性对照(L-NMMA: 32.83 μM)具有较好的抗炎活性,IC50值在10.68 ~ 28.05 μM之间。此外,1通过下调NF-κB和MAPK通路的表达发挥抗炎作用。此外,化合物21和22通过抑制MptpA、MptpB和PTP1B表现出明显的抗结核活性。分子对接结果表明,化合物22与PTP1B活性腔深度结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
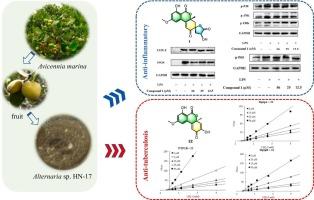
Alterisocoumarins A-M: Isocoumarins with anti-inflammatory and anti-tuberculosis activities from a mangrove endophytic fungus Alternaria sp. HN-17
Fifteen new isocoumarin derivatives 1–3, (±)-4, (±)-5, 6–10, (+)-11, (+)-12, and 13, together with eleven known analogues (−)-11, (−)-12, and 14–22 were isolated from the mangrove endophytic fungus Alternaria sp. HN-17. Their structures were unambiguously established by 1D/2D NMR and HRESIMS spectra, and electronic circular dichroism (ECD) calculations. Alterisocoumarin A (1) possesses a rare 6/6/6/5 tetracyclic skeleton featuring a γ-butyrolactone-fused dibenzo-α-pyrone core, while 2 and 3 have an unusual chlorinated furan ring system. In bioassay, compounds 1, (−)-4, 10, and 18 displayed good anti-inflammatory activity with IC50 values ranging from 10.68 to 28.05 μM than positive control (L-NMMA: 32.83 μM). Moreover, 1 exerts anti-inflammatory effects by down-regulated expression the NF-κB and MAPK pathways. In addition, compounds 21 and 22 showed notable anti-tuberculosis activities by inhibiting MptpA, MptpB and PTP1B. The molecular docking results showed that compound 22 binds deeply in the active cavity of PTP1B.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


