TIM3通过抑制雄性小鼠TRAF6/NF-κB通路减轻吗啡抗痛觉性耐受性和小胶质神经炎症
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
慢性吗啡给药经常导致抗痛觉耐受性的发展,在慢性疼痛管理中提出了重大挑战。虽然已知小胶质细胞介导与吗啡诱导的抗伤害性耐受相关的神经炎症,但这一过程的分子机制仍不完全清楚。最近的证据表明,T细胞免疫球蛋白结构域和粘蛋白结构域-3 (TIM3)在炎症相关疾病中起着重要的调节作用。在这项研究中,我们研究了TIM3在吗啡抗感觉性耐受中的作用。TIM3的药物阻断加重了吗啡抗痛觉性耐受和相关的痛觉过敏,而脊髓中TIM3的上调显著降低了抗痛觉性耐受的发展和维持。我们发现TIM3负调控慢性吗啡暴露后小胶质细胞介导的神经炎症和神经元凋亡。机制上,TIM3促进肿瘤坏死因子受体相关因子6 (TRAF6)的降解,抑制核因子κB (NF-κB)信号通路的激活。此外,我们发现TRAF6是TIM3减弱吗啡诱导的抗炎耐受性和抑制促炎因子分泌的关键介质。值得注意的是,TIM3与肿瘤坏死因子α-诱导蛋白3 (TNFAIP3)相互作用,增强吗啡刺激的小胶质细胞中k48相关的TRAF6泛素化,从而减轻炎症反应。综上所述,这些发现表明脊髓TIM3通过TNFAIP3/TRAF6/NF-κ b依赖机制调节小胶质细胞炎症反应,从而负向调节吗啡抗痛觉性耐受。本研究强调TIM3是预防慢性疼痛治疗中吗啡抗痛觉性耐受的有希望的治疗靶点。TIM3调节吗啡抗感觉性耐受的机制示意图。TIM3可能通过抑制小胶质细胞介导的神经炎症和神经元凋亡来减轻吗啡抗痛觉性耐受,这与TRAF6/NF-κB通路有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
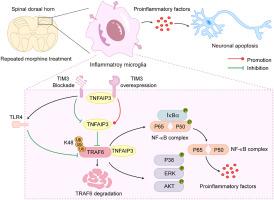
TIM3 attenuates morphine antinociceptive tolerance and microglial neuroinflammation by suppressing the TRAF6/NF-κB pathway in male mice
Chronic morphine administration often leads to the development of antinociceptive tolerance, presenting a significant challenge in the chronic pain management. Although microglia are known to mediate the neuroinflammation associated with morphine-induced antinociceptive tolerance, the molecular mechanisms underlying this process remain incompletely understood. Recent evidence indicates that T cell immunoglobulin domain and mucin domain-3 (TIM3) acts as an important regulator in inflammation-related diseases. In this study, we investigated the role of TIM3 in morphine antinociceptive tolerance. Pharmacological blockade of TIM3 exacerbated morphine antinociceptive tolerance and associated hyperalgesia, whereas upregulation of TIM3 in the spinal cord significantly reduced both the development and maintenance of antinociceptive tolerance. We found that TIM3 negatively regulated microglia-mediated neuroinflammation and neuronal apoptosis following chronic morphine exposure. Mechanistically, TIM3 promoted the degradation of tumor necrosis factor receptor-associated factor 6 (TRAF6) and inhibited the activation of nuclear factor κB (NF-κB) signaling pathways. Furthermore, we identified TRAF6 as a key mediator through which TIM3 attenuated morphine-induced antinociceptive tolerance and suppressed the secretion of proinflammatory factors. Notably, TIM3 interacted with tumor necrosis factor α-induced protein 3 (TNFAIP3) to enhance K48-linked ubiquitination of TRAF6 in morphine-stimulated microglia, thereby mitigating inflammatory responses. Together, these findings suggest that spinal TIM3 negatively modulates morphine antinociceptive tolerance by regulating microglial inflammatory responses through a TNFAIP3/TRAF6/NF-κB-dependent mechanism. This study highlights TIM3 as a promising therapeutic target for preventing morphine antinociceptive tolerance in chronic pain management.
Schematic diagram for the proposed mechanisms of TIM3 regulates morphine antinociceptive tolerance. TIM3 may alleviate morphine antinociceptive tolerance by suppressing microglia-mediated neuroinflammation and neuronal apoptosis, which is associated with the TRAF6/NF-κB pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


