绝经前乳腺癌患者接受抗激素治疗后卵巢功能不完全抑制的评价与处理。
摘要
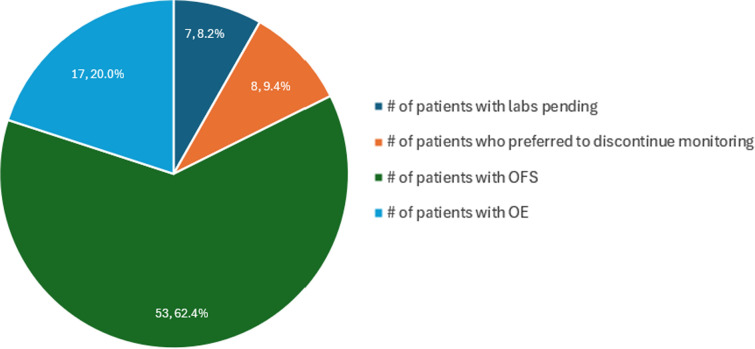
背景:乳腺癌是女性中最常见的恶性肿瘤。他莫昔芬和依西美坦试验(TEXT)和抑制卵巢功能试验(SOFT)是两项具有里程碑意义的研究,表明芳香化酶抑制剂(AI)、依西美坦或他莫昔芬联合卵巢功能抑制(OFS)的辅助治疗可改善绝经前妇女的无病生存(DFS) (Pagani等人in N Engl J Med 371(2):107- 118,2014)。然而,在一些绝经前患者中,关于使用促性腺激素释放激素(GnRH)激动剂的不完全OFS的数据已经出现。SOFT雌激素亚研究(SOFT- est)是SOFT的一项前瞻性亚研究,评估雌二醇(E2)水平,以确定依西美坦和triptorelin治疗的患者是否经历了次优的卵巢功能抑制。在本研究中,17%的患者在每个时间点E2水平大于2.72 pg/mL,确定为不完全性OFS。这项研究提出了一个问题,即在使用GnRH激动剂的女性中,是否应该常规监测E2水平(Bellet等人在《临床肿瘤学杂志》34(14):1584- 1593,2016)。国家综合癌症网络(NCCN)和美国临床肿瘤学会(ASCO)建议监测绝经前患者使用芳香化酶抑制剂(AI)的E2水平,因为担心接受GnRH激动剂治疗的患者的OFS不完全(Burstein等人,journal Clinical Oncology 37(5):423- 438,2019, National Comprehensive Cancer Network)。肿瘤学NCCN临床实践指南:乳腺癌。2.2024版。www.nccn.org)。本研究的目的是根据NCCN和ASCO的建议,制定并实施一项监测Froedtert & MCW E2水平的方案。方法:本研究结合Froedtert & MCW的合作实践协议实施了OFS监测指南。药剂师负责根据指定的方案每月为符合条件的患者订购和监测E2水平。接受AI治疗时E2水平≤2.72 pg/mL或在接受他莫昔芬治疗的同时注射GnRH激动剂时E2水平≤21 pg/mL被定义为完全OFS。回顾性分析E2水平,以评估患者是否有不完全性OFS,以及是否根据这些水平改变了临床管理。主要终点是根据E2监测方案确定的患者在18个月期间连续两次未达到OFS水平的比例。标称数据比较使用卡方或Fisher精确检验,连续数据比较使用Mann Whitney U或学生t检验(如适用)。结果:在分析时共回顾了85例患者。53名患者(62.4%)达到完全OFS(连续3次E2水平在目标范围内),相比之下,17名患者(20%)表现为不完全OFS。7例患者(8.2%)没有足够的连续水平,表明OFS完全或不完全,E2实验室监测仍在继续。8例患者(9.4%)拒绝实验室监测。在17例不完全OFS患者中,14例接受了GnRH激动剂的改变,以达到完全OFS。在这14例患者中,7例成功达到OFS, 5例继续经历不完全的OFS, 2例选择拒绝未来的监测。与干预后完全OFS组相比,持续不完全OFS组更多的患者接受化疗naïve (p = 0.010)。结论:我们的研究表明,有必要检查E2水平来评估不完全OFS,并且改变GnRH激动剂可以提高一些患者的完全OFS率。此外,我们的研究表明化疗naïve患者经历更多不完全OFS;然而,需要进一步的数据来验证这些结果。
Background: Breast cancer is the most common malignancy among females. The Tamoxifen and Exemestane Trial (TEXT) and the Suppression of Ovarian Function Trial (SOFT) were two landmark studies, which showed adjuvant therapy with the aromatase inhibitor (AI), exemestane, or tamoxifen combined with ovarian function suppression (OFS) improved disease-free survival (DFS) among premenopausal women (Pagani et al. in N Engl J Med 371(2):107-118, 2014). However, data has since emerged regarding incomplete OFS with Gonadotropin-releasing hormone (GnRH) agonists in some premenopausal patients. The SOFT Estrogen Substudy (SOFT-EST) was a prospective substudy of SOFT, which evaluated estradiol (E2) levels to determine if patients on exemestane and triptorelin experienced suboptimal ovarian function suppression. In this study, 17% of patients had an E2 level greater than 2.72 pg/mL at each time point, which was determined to be incomplete OFS. This study called into question whether E2 levels should routinely be monitored in women on GnRH agonists (Bellet et al. in J Clin Oncol 34(14):1584-1593, 2016). The National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) provide recommendations to monitor E2 levels in premenopausal patients on an aromatase inhibitor (AI) due to the concern of incomplete OFS for patients receiving GnRH agonist therapy (Burstein et al. in J Clin Oncol 37(5):423-438, 2019, National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 2.2024. www.nccn.org ). The purpose of our study is to develop and implement a protocol for monitoring E2 levels at Froedtert & MCW based on the NCCN and ASCO recommendations.
Methods: This study implemented an OFS monitoring guideline in combination with a collaborative practice agreement at Froedtert & MCW. Pharmacists were responsible for ordering and monitoring E2 levels monthly on eligible patients based on a specified protocol. An E2 level ≤ 2.72 pg/mL while receiving an AI or ≤ 21 pg/mL while receiving tamoxifen in addition to a GnRH agonist injection was defined as complete OFS. E2 levels were retrospectively analyzed to evaluate if patients had incomplete OFS and if clinical management changed based on those levels. The primary outcome was proportion of patients identified per the E2 monitoring protocol that fail to achieve OFS for two consecutive levels over an 18-month period. Nominal data were compared using the chi-squared or Fisher's exact test, and continuous data using the Mann Whitney U or student's t-test, where appropriate.
Results: A total of 85 patients were reviewed at the time of analysis. Fifty-three patients (62.4%) achieved complete OFS (three consecutive E2 levels within goal), compared to 17 patients (20%) demonstrating incomplete OFS. Seven patients (8.2%) did not have enough consecutive levels demonstrating complete or incomplete OFS and E2 lab monitoring continues. Eight patients (9.4%) declined lab monitoring. Out of the 17 patients with incomplete OFS, 14 underwent a change in their GnRH agonist as an intervention aimed at achieving complete OFS. Among these 14 patients, 7 successfully attained OFS, 5 continued to experience incomplete OFS, and 2 opted to decline future monitoring. . More patients were chemotherapy naïve in the continued incomplete OFS group compared to those who had complete OFS after intervention (p = 0.010).
Conclusion: Our study has shown a trend toward the need for checking E2 levels to assess for incomplete OFS and that changing GnRH agonist agent can improve rates of complete OFS for some patients. Additionally, our study has shown that chemotherapy naïve patients experience more incomplete OFS; however, further data is needed to validate these results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


