5-(叠氮多甲基)糠醛1,3-偶极环加成至甲基碱-2,3-二烯酸甲酯的区域选择性合成呋喃取代1,2,3-三唑
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
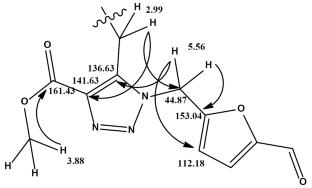
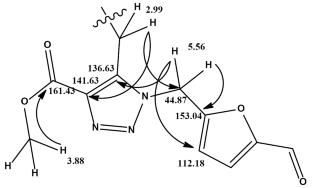
提出了一种以2,3-二烯酸甲酯为原料,区域选择性合成新的1,2,3-三唑衍生物的方法。研究了初始反应物中取代基对反应的区域选择性和立体选择性的影响,并用物理化学方法对合成的化合物进行了表征。5-(叠氮多甲基)糠醛[由5-(氯甲基)糠醛制备]的区域选择性1,3-偶极环加成到含有环亚胺片段的碱-2,3-二烯酸盐,在甲苯中得到取代的1,2,3-三唑,糠醛基和酯基之间的距离最大,从而最大限度地减少了空间排斥。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Regioselective Synthesis of Furfuryl-Substituted 1,2,3-Triazoles by 1,3-Dipolar Cycloaddition of 5-(Azidomethyl)furfural to Methyl Alka-2,3-dienoates
A procedure has been proposed for the regioselective synthesis of new 1,2,3-triazole derivatives from methyl alka-2,3-dienoates. The effect of substituents in the initial reactants on the regio- and stereoselectivity of the reaction has been studied, and the synthesized compounds have been characterized by physicochemical methods. Regioselective 1,3-dipolar cycloaddition of 5-(azidomethyl)furfural [prepared from 5-(chloromethyl)furfural] to alka-2,3-dienoates containing a cyclic imide fragment in toluene afforded substituted 1,2,3-triazoles with maximum remoteness of the furfuryl and ester groups from each other, which minimizes steric repulsion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


