(1,2,4-三嗪基)呋喃唑的合成
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
4-甲基和4-氨基-1,2,5-恶二唑-3-碳肼酰胺与乙二醛反应得到相应的二聚体产物N ',N″-(乙烷-1,2-二乙基)二(4-R-1,2,5-恶二唑-3-碳肼酰胺)(R = Me, NH2),而相同的底物与其他1,2-二羰基化合物,如苯和茚三酮反应得到目标3-(4-R-1,2,5-恶二唑-3-基)-1,2,4-三嗪衍生物。产物的结构经红外、核磁共振和x射线分析证实。所合成的化合物有望作为设计具有不同活性的药理活性化合物的支架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
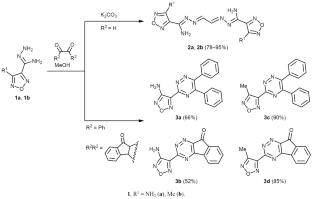
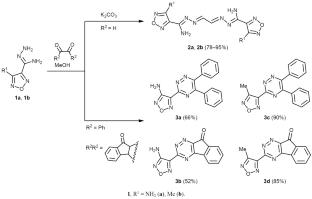
Synthesis of (1,2,4-Triazinyl)furazans
4-Methyl- and 4-amino-1,2,5-oxadiazole-3-carbohydrazonamides reacted with glyoxal to give the corresponding dimeric products, N′,N″-(ethane-1,2-diylidene)bis(4-R-1,2,5-oxadiazole-3-carbohydrazonamides) (R = Me, NH2), whereas the reaction of the same substrates with other 1,2-dicarbonyl compounds, such as benzil and ninhydrin afforded target 3-(4-R-1,2,5-oxadiazol-3-yl)-1,2,4-triazine derivatives. The product structure was confirmed by IR and NMR spectra and X-ray analysis. The synthesized compounds are promising as scaffolds for the design of pharmacologically active compounds with different kinds of activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


