新的手性双环吡咯烷阻断剂用于Nirmatrelvir及其类似物
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
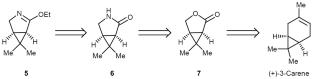
(1R,5S)-2-乙氧基-6,6-二甲基-3- azabicycloo[3.1.0]己二烯很容易从(+)-3-蒈烯中获得,被认为是合成Nirmatrelvir及其相关化合物的关键组成部分。它是由(1R,5S)-6,6-二甲基-3-氮杂环[3.1.0]己烷-2- 1与四氟硼酸三乙基氧鎓烯醇化而得。本文章由计算机程序翻译,如有差异,请以英文原文为准。
New Chiral Bicyclic Pyrrolidine Block for Nirmatrelvir and Its Analogues
(1R,5S)-2-Ethoxy-6,6-dimethyl-3-azabicyclo[3.1.0]hex-2-ene easily available from (+)-3-carene has been proposed as a key building block for the synthesis of Nirmatrelvir and related compounds. It has been obtained by enol ethylation of (1R,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-one with triethyloxonium tetrafluoroborate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


