仲膦硫族化合物与巯基烷醇的化学选择性氧化交偶联:s-羟基烷基膦硫代酸盐的合成
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在CCl4/Et3N的存在下,以等摩尔的反应物比(20-25℃,2-7 h)氧化交叉偶联得到了相应的s-羟基烷基膦硫代硫代酸盐,其化学选择性高,分离收率为76-89%。本文章由计算机程序翻译,如有差异,请以英文原文为准。

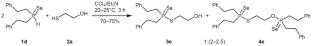
Chemoselective Oxidative Cross-Coupling of Secondary Phosphine Chalcogenides with Mercaptoalkanols: Synthesis of S-Hydroxyalkyl Phosphinochalcogenothioates
Oxidative cross-coupling of secondary phosphine chalcogenides with mercaptoalkanols in the presence of CCl4/Et3N at an equimolar reactant ratio under mild conditions (20–25°C, 2–7 h) afforded the corresponding S-hydroxyalkyl phosphinochalcogenothioates with high chemoselectivity in 76–89% isolated yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


