蒽醌修饰剂KA-4c触发内质网应激抑制三阴性乳腺癌细胞的机制及靶点研究
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-08-18
DOI:10.1016/j.bbadis.2025.168020
引用次数: 0
摘要
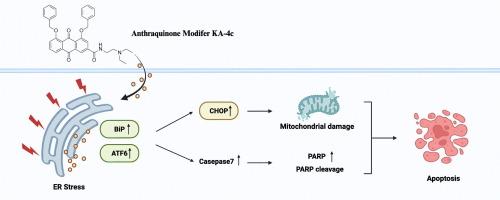
由于缺乏靶向治疗,三阴性乳腺癌(TNBC)预后差,转移和复发率高。因此,确定TNBC治疗的有效靶点仍然是一个重要的临床挑战。在这项研究中,我们研究了蒽醌修饰剂KA-4c的潜在抗癌机制和靶点。采用苏木精/伊红(HE)、ER-Tracker™Red和Mito-Tracker™Green染色,然后通过透射电子显微镜(TEM)研究KA-4c对TNBC细胞形态的影响。流式细胞术检测ka -4c诱导的细胞凋亡,western blotting检测细胞凋亡相关蛋白。采用药物亲和响应靶稳定性(dart)、液相色谱-串联质谱(LC-MS/MS)、生物信息学、细胞热移分析(CETSA)和RNA干扰等方法对KA-4c的靶蛋白进行鉴定。结果显示,KA-4c处理的MDA-MB231和MDA-MB468细胞内质网和线粒体的细胞质空泡化增加。此外,KA-4c通过上调CHOP和caspase7的表达,诱导PARP切割,增强MDA-MB231和MDA-MB468细胞的凋亡。DARTS结果显示,KA-4c通过结合ATF6激活内质网蛋白加工信号通路,使其抵抗蛋白酶水解。CETSA结果表明,KA-4c以浓度依赖性的方式增强ATF6蛋白的表达。RNA干扰结果表明,沉默ATF6可以有效抑制CHOP的上调。综上所述,KA-4c通过靶向ATF6,破坏线粒体,诱导TNBC细胞凋亡,激活内质网蛋白加工信号通路。因此,ATF6代表了KA-4c的潜在靶点,也是TNBC的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic and target study of anthraquinone modifier KA-4c triggering endoplasmic reticulum stress to inhibit triple-negative breast cancer cells
Triple-negative breast cancer (TNBC) is associated with poor prognosis and high rates of metastasis and recurrence owing to lack of targeted therapies. Therefore, identifying effective targets for TNBC therapy remains an important clinical challenge. In this study, we examined the potential anticancer mechanisms and targets of the anthraquinone modifier KA-4c. Hematoxylin/eosin (HE), ER-Tracker™ Red, and Mito-Tracker™ Green staining followed by transmission electron microscopy (TEM) were performed to study the effect of KA-4c on TNBC cell morphology. KA-4c-induced apoptosis was detected using flow cytometry, and apoptosis-related proteins were analyzed using western blotting. Drug affinity-responsive target stability (DARTS), liquid chromatography tandem mass spectrometry (LC-MS/MS), bioinformatics, cell thermal shift analysis (CETSA), and RNA interference were used to identify the target protein of KA-4c. The results revealed increased cytoplasmic vacuolation from the endoplasmic reticulum and mitochondria in MDA-MB231 and MDA-MB468 cells treated with KA-4c. Furthermore, KA-4c enhanced MDA-MB231 and MDA-MB468 cell apoptosis by upregulating CHOP and caspase7 expression, and inducing PARP cleavage. DARTS results revealed that KA-4c activates the ER protein-processing signaling pathway by binding to ATF6 and rendering it resistant to protease hydrolysis. CETSA results demonstrated that KA-4c enhances ATF6 protein expression in a concentration-dependent manner. The results of RNA interference indicated that silencing ATF6 could effectively inhibit the upregulation of CHOP. In conclusion, KA-4c activates the ER protein-processing signaling pathway by targeting ATF6, damaging mitochondria, and inducing TNBC cell apoptosis. Thus, ATF6 represents a potential target of KA-4c, and a therapeutic target for TNBC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


