河口如何调节铜(Cu)和镉(Cd)对海水的河流通量
IF 3.6
2区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
向沿海海洋输出溶解铜(Cu)和镉(Cd)对维持海洋生物生产力具有重要意义。河流输入是Cu和Cd的主要来源,分别为8.3 ~ 10.4亿mol /yr和1000 ~ 3000万mol /yr。研究表明,河口过程可能会增加河流输出通量,最多增加20%的Cu和271%的Cd。为了了解Cu和Cd河口循环的潜在机制,我们研究了恒河(Hooghly)河口,这条季风主导的热带河流以高泥沙比为特征,作为研究大规模全球河口过程的自然实验室。从河流悬浮沉积物的可交换(Cd)和/或无定形氧化物(Cu)相中金属的再活化被确定为有助于其河口生产的主要过程。此外,在贫流季节,浅孔水排放的Cu输入调节了高盐度带的溶解Cu浓度。稳态质量平衡模型表明,河口溶解Cu和Cd浓度的变化是溶解无机络合的函数,而溶解无机络合又受pH、碳酸氢盐离子浓度和盐度(含氯度)的变化调节。关键的发现是,在中高盐度范围(4 ~ 13‰),溶解的Cu和Cd相对于保守的河水-海水混合表现出浓度“过剩”,这被金属的反应固相浓度成比例地降低所证实。相对于流入孟加拉湾的河流供应,“过量”浓度导致铜和镉出口分别增加~ 46%和~ 306%。总的来说,这项研究强调了河口过程在调节净河流金属通量到海水中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
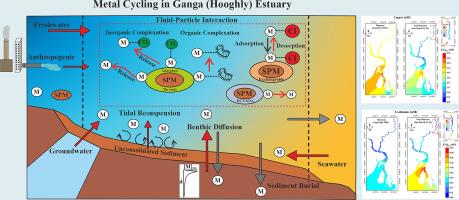
How estuaries modulate the riverine fluxes of copper (Cu) and cadmium (Cd) to seawater
The export of dissolved copper (Cu) and cadmium (Cd) to coastal ocean is important for sustaining marine biological productivity. Riverine input is the dominant source of Cu and Cd to seawater, which supplies about 830–1040 million moles/yr and 10–30 million moles/yr respectively. We demonstrate that estuarine processes may augment the riverine export fluxes by a maximum 20 % for Cu and 271 % for Cd. To understand the underlying mechanism of estuarine cycling of Cu and Cd, we studied the Ganga (Hooghly) River estuary, a monsoon-dominated tropical river characterized by a high sediment-to-water ratio, serving as a natural laboratory to investigate large-scale global estuarine processes. The remobilization of metals from exchangeable (Cd) and/or amorphous oxide (Cu) phases of the riverine suspended sediments are identified to be the primary processes contributing to their estuarine production. In addition, during the lean flow season, Cu input from shallow porewater discharge modulates dissolved Cu concentration in the high salinity zone. Steady state mass balance models reveal that the variation in dissolved Cu and Cd concentrations in the estuary is a function of dissolved inorganic complexation, which in turn is modulated by changes in pH, bicarbonate-ion concentrations and salinity (chlorinity). The key finding is that the dissolved Cu and Cd in the mid to high salinity range (4–13 ‰) exhibit a concentration “excess” relative to conservative river water-seawater mixing which is corroborated by a proportionate decrease in reactive solid phase concentration of the metals. The “excess” concentrations result in ∼46 % and ∼ 306 % rise in Cu and Cd export, relative to the riverine supply, to the Bay of Bengal. Overall, this study underscores the importance of estuarine processes in modulating net riverine metal fluxes to seawater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Geology
地学-地球化学与地球物理
CiteScore
7.20
自引率
10.30%
发文量
374
审稿时长
3.6 months
期刊介绍:
Chemical Geology is an international journal that publishes original research papers on isotopic and elemental geochemistry, geochronology and cosmochemistry.
The Journal focuses on chemical processes in igneous, metamorphic, and sedimentary petrology, low- and high-temperature aqueous solutions, biogeochemistry, the environment and cosmochemistry.
Papers that are field, experimentally, or computationally based are appropriate if they are of broad international interest. The Journal generally does not publish papers that are primarily of regional or local interest, or which are primarily focused on remediation and applied geochemistry.
The Journal also welcomes innovative papers dealing with significant analytical advances that are of wide interest in the community and extend significantly beyond the scope of what would be included in the methods section of a standard research paper.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


