基于双铁下垂诱导的微针贴片用于增强化疗/光热联合治疗三阴性乳腺癌
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
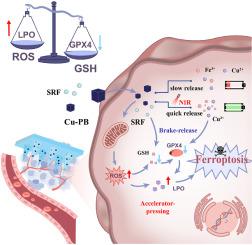
三阴性乳腺癌(TNBC)仍然是一种难治性乳腺癌亚型,因为它对各种治疗策略具有耐药性。在这项研究中,我们引入了一种“刹车释放和加速器按压”的方法来设计一种微针贴片,该贴片嵌入了掺杂铜的Prussian nanoparticles (Cu-PB)和铁凋亡诱导剂sorafenib (SRF),用于提高化学动力学(CDT)/光热(PTT)联合治疗TNBC。经皮插入后,溶解的微针迅速分解并促进SRF的释放。在温和的外部光照下,铜离子(Cu2+)和铁离子(Fe3+)从Cu-PB中释放出来。Cu2+的直接螯合和SRF的间接抑制共同减弱谷胱甘肽过氧化物酶4 (GPX4)的酶功能,破坏细胞氧化还原平衡(称为“制动释放”策略)。Cu2+和Fe3+离子的释放引发肿瘤细胞内的Fenton/Fenton样反应,进一步产生羟基自由基并提高活性氧(ROS)浓度(被称为“加速器按压”策略)。这种压倒性的ROS积累,加上由此产生的脂质过氧化物(LPO)的清除受损,最终引发了强烈的铁下垂细胞死亡反应。总之,本研究提出了一种基于双铁下垂诱导TNBC的创新组合治疗策略,这意味着为开发以铁下垂为中心的治疗这种侵袭性乳腺癌亚型提供了一个有希望的治疗平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual-ferroptosis induction-based microneedle patches for enhanced chemodynamic/photothermal combination therapy against triple-negative breast cancer
Triple-negative breast cancer (TNBC) remains a refractory subtype of breast cancer due to its resistance to various therapeutic strategies. In this study, we introduce a “brake-release and accelerator-pressing” approach to engineer a microneedle patch embedded with copper-doped Prussian blue nanoparticles (Cu-PB) and the ferroptosis inducer sorafenib (SRF) for raised chemodynamic (CDT)/photothermal (PTT) combination therapy against TNBC. Upon transdermal insertion, the dissolving microneedles swiftly disintegrate and facilitate the release of SRF. Under gentle external light exposure, copper ions (Cu2+) and iron ions (Fe3+) were liberated from Cu-PB. The direct chelation of Cu2+ and the indirect suppression by SRF, collectively attenuate glutathione peroxidase 4 (GPX4) enzymatic function, destabilizing the cellular redox equilibrium (referred to as the “brake-release” strategy). The release of Cu2+ and Fe3+ ions instigates a Fenton/Fenton-like reaction within tumor cells, further yielding hydroxyl radicals and elevating reactive oxygen species (ROS) concentrations (referred to as the “accelerator-pressing” strategy). This overwhelming ROS accumulation, coupled with the impaired clearance of resultant lipid peroxides (LPO), ultimately triggers a robust ferroptosis cell death response. In summary, this study presents an innovative combinatorial therapeutic strategy based on dual-ferroptosis induction for TNBC, implying a promising therapeutic platform for developing ferroptosis-centered treatments for this aggressive breast cancer subtype.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


