小样本多单位制药生产中质量预测和诊断的ai集成IQPD框架:从经验驱动向数据驱动的制造推进
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
制药行业在复杂的多阶段过程的质量数字化方面面临挑战,特别是在小样本系统中。本研究利用4个生产单位从原料到成品的4年数据,开发了智能质量预测与诊断(IQPD)框架,并将其应用于同仁堂牛黄清心丸。在此框架下,提出了一种新的路径增强双集成质量预测模型(PeDGAT),该模型结合图关注网络和路径信息对单元间的远程和顺序依赖进行编码。此外,双集成策略提高了模型在小样本中的稳定性。与全球传统模型相比,PeDGAT取得了最先进的结果,在三个指标上的预测精度和稳定性平均提高了13.18%和87.67%。此外,利用灰色关联分析和专家知识的更深入的诊断模型减少了对大样本的依赖,提供了跨单元属性关系的全景视图,并提高了流程透明度。最后,IQPD框架集成到人-网络-物理系统中,为同仁堂的牛黄清心丸提供了更快的决策和实时质量调整,该产品年销售额超过1亿元人民币。这有助于从经验驱动型制造向数据驱动型制造的转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
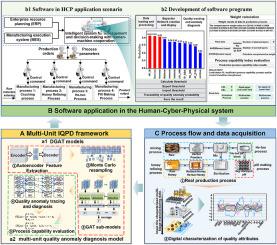
AI-integrated IQPD framework of quality prediction and diagnostics in small-sample multi-unit pharmaceutical manufacturing: Advancing from experience-driven to data-driven manufacturing
The pharmaceutical industry faces challenges in quality digitization for complex multi-stage processes, especially in small-sample systems. Here, an intelligent quality prediction and diagnostic (IQPD) framework was developed and applied to Tong Ren Tang's Niuhuang Qingxin Pills, utilizing four years of data collected from four production units, covering the entire process from raw materials to finished products. In this framework, a novel path-enhanced double ensemble quality prediction model (PeDGAT) is proposed, which combines a graph attention network and path information to encode inter-unit long-range and sequential dependencies. Additionally, the double ensemble strategy enhances model stability in small samples. Compared to global traditional models, PeDGAT achieves state-of-the-art results, with an average improvement of 13.18% and 87.67% in prediction accuracy and stability on three indicators. Additionally, a more in-depth diagnostic model leveraging grey correlation analysis and expert knowledge reduces reliance on large samples, offering a panoramic view of attribute relationships across units and improving process transparency. Finally, the IQPD framework integrates into a Human–Cyber–Physical system, enabling faster decision-making and real-time quality adjustments for Tong Ren Tang's Niuhuang Qingxin Pills, a product with annual sales exceeding 100 million CNY. This facilitates the transition from experience-driven to data-driven manufacturing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


