口服活性、安全、广谱联苯- dapy衍生物作为高效HIV-1非核苷类逆转录酶抑制剂的生物等构驱动设计
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究旨在确定具有强抗hiv -1活性和理想的药物样特性的理想候选药物。我们的努力涉及到生物同位策略的实施,导致发现了一组含卤素的联苯二酰嘧啶作为有效的HIV-1非核苷逆转录酶抑制剂。值得注意的是,化合物A12对WT HIV-1 (EC50 = 1.9 nmol/L)和7个突变株(EC50 = 1.7-157 nmol/L)均表现出卓越的疗效,超过了先导化合物6,与曲维碱相当。此外,该类似物的不良反应最小,细胞毒性显著降低(CC50 = 195 μmol/L),选择性指数高(SI = 102,608),优于依曲维碱(CC50 = 4.6 μmol/L, SI > 1436)和利匹韦林(CC50 = 3.98 μmol/L, SI = 3989)。对CYP (IC50 = 6.99 ~ 25 μmol/L)和hERG (IC50 > 40 μmol/L)具有较低的抑制作用,与依曲维林和利匹韦林相比具有较强的安全性。单次剂量2 g/kg未见急性毒性或器官病理损伤。此外,A12具有良好的口服生物利用度(F = 29.2%)和较长的消除半衰期(T1/2 = 13.56 h),可以方便地以最小剂量口服给药。这些发现表明A12可以作为一种有希望的HIV治疗候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
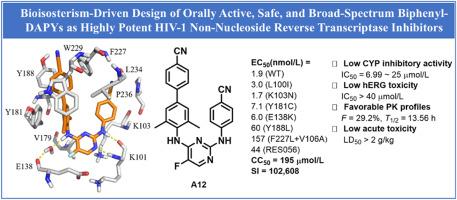
Bioisosterism-driven design of orally active, safe, and broad-spectrum biphenyl-DAPY derivatives as highly potent HIV-1 non-nucleoside reverse transcriptase inhibitors
This study aimed to identify ideal pharmaceutical candidates featuring strong anti-HIV-1 activity and desirable drug-like characteristics. Our endeavor involved the implementation of a bioisosterism strategy, leading to the discovery of an assemblage of halogen-containing biphenyl-diarylpyrimidines as potent HIV-1 non-nucleoside reverse transcriptase inhibitors. Notably, compound A12 demonstrated exceptional efficacy against both WT HIV-1 (EC50 = 1.9 nmol/L) and seven mutant strains (EC50 = 1.7–157 nmol/L), surpassing that of the lead compound 6 and comparable to etravirine. Furthermore, this analog exhibited minimal adverse effects with significantly reduced cytotoxicity (CC50 = 195 μmol/L) and a high selectivity index (SI = 102,608), superior to those of etravirine (CC50 > 4.6 μmol/L, SI > 1436) and rilpivirine (CC50 = 3.98 μmol/L, SI = 3989). It displayed low inhibition of CYP (IC50 = 6.99–25 μmol/L) and hERG (IC50 > 40 μmol/L), indicating a safer profile compared to etravirine and rilpivirine. No acute toxicity or organ pathological damage was observed at a single dose of 2 g/kg. Additionally, A12 exhibited favorable oral bioavailability (F = 29.2%) and an extended elimination half-life (T1/2 = 13.56 h), enabling convenient oral administration at minimal doses. These findings indicated that A12 could serve as a promising drug candidate for HIV treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


