人工间充质干细胞细胞外囊泡通过靶向重塑脑微血管内皮细胞增强缺血性卒中治疗
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
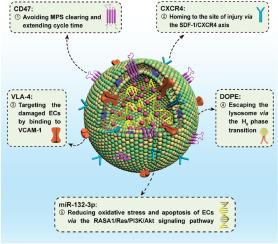
缺血性中风是全世界致残和死亡的主要原因。血脑屏障(BBB)是缺血性中风后的第一道防线。脑微血管内皮细胞(BMECs)功能障碍引起血脑屏障破坏是引发中枢神经系统继发性损伤的关键事件,其中血源性液体和免疫细胞穿透脑实质,引起脑水肿和炎症反应,并进一步加重脑损伤。在这里,我们通过将MSC膜蛋白整合到脂质体双层中,开发了一种新型的人工间充质干细胞(MSC)细胞外囊泡,该囊泡包被miR-132-3p,对BMECs具有保护作用。人工细胞外囊泡(MSCo/miR-132-3p)具有低免疫原性,可减少单核吞噬系统(MPS)的非特异性清除,可靶向缺血损伤的bmec。在内化到受损的bmec后,MSCo/miR-132-3p通过1,2-二油基-sn-甘油-3-磷酸乙醇胺(DOPE)的HII期转变逃离溶酶体,并通过调节RASA1/RAS/PI3K/AKT信号通路降低细胞活性氧(ROS)和凋亡水平。在短暂性大脑中动脉闭塞(tMCAO)模型中,MSCo/miR-132-3p靶向受损脑区域(12 h时普通脂质体的积累量约为9倍),减少脑血管破坏,保护血脑屏障完整性,减少梗死体积(从44.95%降至6.99%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Artificial mesenchymal stem cell extracellular vesicles enhanced ischemic stroke treatment through targeted remodeling brain microvascular endothelial cells
Ischemic stroke is the leading cause of disability and mortality worldwide. The blood‒brain barrier (BBB) is the first line of defense after ischemic stroke. Disruption of the BBB induced by brain microvascular endothelial cells (BMECs) dysfunction is a key event that triggers secondary damage to the central nervous system, where blood-borne fluids and immune cells penetrate the brain parenchyma, causing cerebral edema and inflammatory response and further aggravating brain damage. Here, we develop a novel artificial mesenchymal stem cell (MSC) extracellular vesicles by integrating MSC membrane proteins into liposomal bilayers, which encapsulated miR-132-3p with protective effects on BMECs. The artificial extracellular vesicles (MSCo/miR-132-3p) had low immunogenicity to reduce non-specific clearance by the mononuclear phagocytosis system (MPS) and could target ischemia-injured BMECs. After internalization into the damaged BMECs, MSCo/miR-132-3p escaped the lysosomes via the HII phase transition of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and decreased cellular reactive oxygen species (ROS) and apoptosis levels by regulating the RASA1/RAS/PI3K/AKT signaling pathway. In the transient middle cerebral artery occlusion (tMCAO) models, MSCo/miR-132-3p targeted impaired brain regions (approximately 9 times the accumulation of plain liposomes at 12 h), reduced cerebral vascular disruption, protected BBB integrity, and decreased infarct volume (from 44.95% to 6.99%).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


