含非规范辅助因子的工程血红蛋白趋向于人工金属酶。
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
血红蛋白已成为构建人工金属酶的多功能支架。通过随机和/或位点饱和诱变的定向进化,这些蛋白质可以重新用于催化非生物转化。通过引入非典型分子组分,可以进一步扩大其催化范围。一种方法是将非规范氨基酸残基(如甲基组氨酸)结合到蛋白质支架中。另一种策略是用合成辅因子代替天然血红素。虽然天然血红素辅助因子通常仅限于卟啉,但合成化学已经能够获得各种卟啉衍生物和具有不同核心结构和外围功能的人工卟啉。这篇综述强调了最近在设计这种非规范辅助因子和工程互补蛋白突变体以实现具有挑战性的转化方面的努力,包括C-H羟基化/胺化和烯烃环丙烷化。通过整合非规范辅助因子来扩展血红蛋白的化学空间,是开发具有新颖和有价值催化功能的人工金属酶的一个有希望的方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
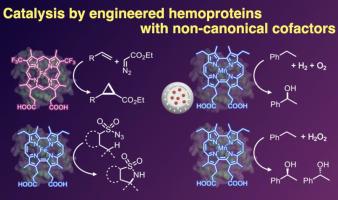
Engineered hemoproteins containing non-canonical cofactors toward artificial metalloenzymes
Hemoproteins have emerged as versatile scaffolds for the construction of artificial metalloenzymes. Through directed evolution via random and/or site-saturation mutagenesis, these proteins can be repurposed to catalyze abiological transformations. Their catalytic scope can be further expanded by introducing non-canonical molecular components. One approach involves the incorporation of non-canonical amino acid residues, such as methylhistidine, into the protein scaffold. Another strategy replaces the native heme with synthetic cofactors. While natural heme cofactors are generally restricted to porphyrins, synthetic chemistry has enabled access to a variety of porphyrin derivatives and artificial porphyrinoids with diverse core structures and peripheral functionalities. This review highlights recent efforts in designing such non-canonical cofactors and engineering complementary protein mutants to achieve challenging transformations, including C–H hydroxylation/amination and olefin cyclopropanation. Expanding the chemical space of hemoproteins through the integration of non-canonical cofactors represents a promising direction toward artificial metalloenzymes with novel and valuable catalytic functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


