GPR41和GPR43调节啮齿动物胰腺α-细胞功能和生长
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的:虽然SCFA受体GPR41和GPR43调节β细胞胰岛素分泌,但它们在α细胞中的作用尚不清楚,尽管2型糖尿病(T2D)患者存在高胰高血糖素血症。因此,本研究旨在探讨合成GPR41和GPR43激动剂对α-细胞生理和营养刺激反应的调节能力。方法利用αTC1.9细胞和原代大鼠胰岛,研究高脂喂养(HFD)和哺乳期(L)相关生理和胰岛素抵抗条件下,SCFA受体在胰高血糖素表达和分泌中的作用。采用特异性激动剂AR420626 (AR)和(S)-2-(4-氯苯基)-3,3-二甲基- n -(5-苯基噻唑-2-基)丁酰胺(PA)研究其作用机制。结果胰岛组织组织学和流式细胞术分析表明,GPR41和GPR43定位于α-细胞。gpr41 -受体激动剂AR或gpr43 -受体激动剂PA处理αTC1.9细胞后,低糖时Gcg表达增加,胰高血糖素分泌增加,高糖时AR增强胰高血糖素释放。这种效果在孤立的胰岛中得到了再现,证明百日咳毒素对两种激动剂的敏感性。hfd喂养的动物表现为葡萄糖耐受不良、早期空腹高胰高血糖素血症和胰岛对葡萄糖的抵抗,抑制胰高血糖素分泌,同时胰岛Gpr41/43表达增强。用合成激动剂刺激HFD胰岛进一步增加Gcg的表达。泌乳期间胰腺GPR41 /43水平也被短暂诱导,尽管只有GPR41激活泌乳大鼠胰岛通过g - αi和α-细胞复制上调Gcg表达。结论这些发现表明GPR41是一个有希望的治疗靶点,可以调节高胰高血糖素血症和改善t2dm的血糖控制,支持其在糖尿病干预策略中的翻译相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
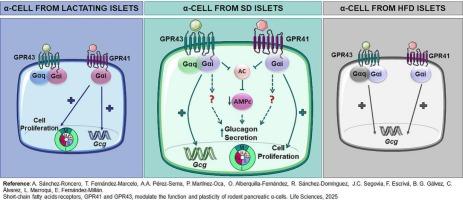
GPR41 and GPR43 modulate rodent pancreatic α-cell function and growth
Objective
While SCFA receptors GPR41 and GPR43 regulate β-cell insulin secretion, their role in α-cells remains unknown despite hyperglucagonemia in type 2 diabetes (T2D). Thus, the current study aims to investigate the ability of synthetic GPR41 and GPR43 agonists to modulate α-cell physiology and responsiveness to nutrient challenge.
Methods
Using αTC1.9 cells and primary rat islets we investigated the role of SCFA receptors in glucagon expression and secretion under physiological and insulin resistant conditions associated with high-fat feeding (HFD) and lactation (L). The specific agonists AR420626 (AR) and (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl) butanamide (PA) were employed to study the mechanisms involved.
Results
Histological and flow cytometry analysis of islets demonstrated that GPR41 and GPR43 localized in α-cells. Treatment of αTC1.9 cells with the GPR41-agonist AR or GPR43-agonist PA increased Gcg expression and glucagon secretion at low glucose, while AR also potentiated glucagon release at high glucose. This effect was recapitulated in isolated islets demonstrating pertussis toxin sensitivity for both agonist effects. HFD-fed animals showed glucose intolerance, early fasting hyperglucagonemia and islet resistance to glucose inhibition of glucagon secretion together with enhanced expression of islet Gpr41/43. Stimulation of HFD islets with the synthetic agonists further increased Gcg expression. Pancreatic Gpr41/43 levels were also transiently induced during lactation although only GPR41 activation of lactating rat islets up-regulated Gcg expression via Gαi and α-cell replication.
Conclusions
These findings position GPR41 as a promising therapeutic target for modulating hyperglucagonemia and improving glycemic control in T2D, supporting its translational relevance in diabetes intervention strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


