超增强子驱动的PRSS27上调通过激活PI3K/AKT通路,在肺腺癌中减弱氧化应激和细胞凋亡。
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
肺腺癌(LUAD)仍然是世界范围内癌症相关死亡的主要原因。本研究旨在阐明超级增强子(SEs)驱动蛋白酶丝氨酸27 (PRSS27)持续致癌表达的机制,PRSS27是LUAD中一种以前未被发现的基因。综合多组学分析,包括组蛋白H3赖氨酸27乙酰化(H3K27ac)切割和标记测序(CUT&Tag-seq), RNA测序(RNA-seq)和公共数据集,确定PRSS27是luad特异性se调控基因,高表达与不良预后相关。功能分析表明,抑制SEs (JQ-1和i-BET151)抑制LUAD细胞活力、增殖和集落形成,同时促进细胞凋亡。PRSS27过表达可促进LUAD肿瘤体外和体内生长,降低氧化应激和细胞凋亡,激活PI3K/AKT信号通路。机制上,CRISPR干扰(CRISPRi)、荧光素酶报告基因检测和染色质免疫沉淀后的定量PCR (ChIP-qPCR)证实,chr16:2689953-2714630位点的SE区域转录激活了PRSS27。值得注意的是,拯救实验显示PRSS27过表达有效地减轻了抑制剂诱导的SEs氧化应激和凋亡。总的来说,这些发现确定了se驱动的PRSS27激活是一种新的致癌基因,通过调节PI3K/AKT信号通路促进LUAD进展,突出了其作为治疗靶点的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
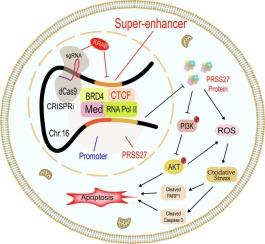
Upregulation of PRSS27 driven by super-enhancers attenuates oxidative stress and apoptosis via activating PI3K/AKT pathway in lung adenocarcinoma
Lung adenocarcinoma (LUAD) remains a leading cause of cancer-related mortality worldwide. This study aims to elucidate the mechanisms by which super-enhancers (SEs) drive persistent oncogenic expression of protease serine 27 (PRSS27)—a previously uncharacterised gene in LUAD. Integrated multi-omics analyses, including histone H3 lysine 27 acetylation (H3K27ac) Cleavage Under Targets and Tagmentation sequencing (CUT&Tag-seq), RNA sequencing (RNA-seq), and public datasets, identified PRSS27 as a LUAD-specific SE-regulated gene, with high expression correlating with poor prognosis. Functional assays demonstrated that inhibition of SEs (JQ-1 and i-BET151) suppressed LUAD cell viability, proliferation, and colony formation while promoting apoptosis. PRSS27 overexpression enhanced LUAD tumour growth in vitro and in vivo, decreased oxidative stress and apoptosis, and activated the PI3K/AKT signalling pathway. Mechanistically, CRISPR interference (CRISPRi), luciferase reporter assays, and chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR) confirmed that the SE region at chr16:2689953-2714630 transcriptionally activated PRSS27. Notably, rescue assays showed that PRSS27 overexpression effectively mitigated inhibitor-induced oxidative stress and apoptosis of SEs. Collectively, these findings identify SE-driven activation of PRSS27 as a novel oncogene that promotes LUAD progression by modulating PI3K/AKT signalling pathway, highlighting its potential as a therapeutic target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


