MRP4缺乏通过cAMP-CREB-CRTC2激活导致脂质代谢失调和脂肪组织炎症。
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
多药耐药相关蛋白4 (MRP4/ABCC4)是一种质膜转运蛋白,在内源性代谢物和外源物的外排中起关键作用。最近的研究也表明MRP4与脂肪形成和脂肪酸代谢有关。我们之前使用MRP4敲除(MRP4-/-)小鼠的工作表明,MRP4缺乏与肥胖和糖尿病的发展之间存在很强的关联。然而,MRP4调控脂肪组织功能的潜在机制尚不清楚。目的:探讨MRP4在高脂高糖(HFHS)饮食条件下脂肪组织功能障碍及代谢调节中的作用。材料和方法:MRP4敲除(MRP4-/-)小鼠和野生型(WT)小鼠分别饲喂常规饲料或HFHS饲料24 周。评估体重、血糖、胆固醇、胰岛素分泌、葡萄糖耐量和胰岛素敏感性。测量了身体成分、体力活动和能量消耗。分析附睾脂肪组织(EA)的基因表达(脂肪生成、脂肪生成、纤维化和炎症标志物)、组织学、cAMP水平以及磷酸化CREB (P-CREB)和CRTC2的蛋白表达。主要发现:MRP4-/-小鼠比WT对照组体重增加更大,即使是在鼠粮饮食中。在HFHS条件下,他们表现出加剧的代谢功能障碍,包括血糖和胆固醇水平升高,脂肪增加,脂肪细胞肥大和瘦素水平改变。这些小鼠还表现出胰岛素分泌受损、葡萄糖耐量降低和胰岛素敏感性降低。身体成分分析显示脂肪量增加,瘦肉量减少,水潴留增加。MRP4-/-小鼠在EA中表现出身体活动减少、能量消耗改变、脂肪生成、纤维化和炎症基因上调。组织学分析证实了炎症和纤维化。cAMP水平升高,P-CREB和CRTC2表达增加,表明cAMP- creb -CRTC2信号通路激活。意义:MRP4缺乏通过激活cAMP-CREB-CRTC2信号轴促进脂肪组织炎症、纤维化和代谢功能障碍。这些发现揭示了MRP4在维持脂肪组织稳态和防止饮食引起的代谢疾病方面的一种新的调节作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
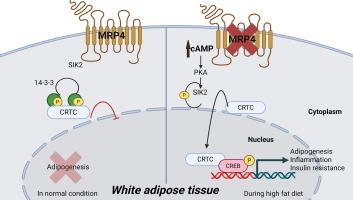
MRP4 deficiency drives lipid metabolism dysregulation and adipose tissue inflammation through cAMP-CREB-CRTC2 activation
Multidrug resistance-associated protein 4 (MRP4/ABCC4), a plasma membrane transporter, plays a critical role in the efflux of endogenous metabolites and xenobiotics. Recent studies have also implicated MRP4 in adipogenesis and fatty acid metabolism. Our previous work using MRP4 knockout (MRP4-/-) mice demonstrated a strong association between MRP4 deficiency and the development of obesity and diabetes. However, the underlying mechanisms through which MRP4 regulates adipose tissue function remain unclear.
Aim
To investigate the role of MRP4 in adipose tissue dysfunction and metabolic regulation under high-fat, high-sucrose (HFHS) diet conditions.
Materials and methods
MRP4 knockout (MRP4-/-) and wild-type (WT) mice were fed either a regular chow or HFHS diet for 24 weeks. Body weight, plasma glucose, cholesterol, insulin secretion, glucose tolerance, and insulin sensitivity were assessed. Body composition, physical activity, and energy expenditure were measured. Epididymal adipose tissue (EA) was analyzed for gene expression (adipogenic, lipogenic, fibrotic, and inflammatory markers), histology, cAMP levels, and protein expression of phosphorylated CREB (P-CREB) and CRTC2.
Key findings
MRP4-/- mice showed greater body weight gain than WT controls, even on a chow diet. Under HFHS conditions, they exhibited exacerbated metabolic dysfunction, including elevated glucose and cholesterol levels, increased adiposity, adipocyte hypertrophy, and altered leptin levels. These mice also showed impaired insulin secretion, reduced glucose tolerance, and decreased insulin sensitivity. Body composition analysis revealed higher fat mass, lower lean mass, and increased water retention. MRP4-/- mice displayed reduced physical activity, altered energy expenditure, and upregulation of adipogenic, fibrotic, and inflammatory genes in EA. Histological analysis confirmed inflammation and fibrosis. Elevated cAMP levels, along with increased P-CREB and CRTC2 expression, indicated activation of the cAMP-CREB-CRTC2 signaling pathway.
Significance
MRP4 deficiency promotes adipose tissue inflammation, fibrosis, and metabolic dysfunction through activation of the cAMP-CREB-CRTC2 signaling axis. These findings reveal a novel regulatory role for MRP4 in maintaining adipose tissue homeostasis and protecting against diet-induced metabolic disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


