巨噬细胞和巨噬细胞胞外囊泡通过prdx6介导的线粒体自噬抑制赋予癌症铁凋亡抗性
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
随着肿瘤微环境(tumor microenvironment, TME)在铁下垂的调控中发挥着关键作用,铁下垂已成为癌症治疗中一个很有前景的治疗靶点。尽管巨噬细胞参与TME中的铁下垂调节,但其潜在机制尚不清楚。在这项研究中,我们证明了巨噬细胞在各种肿瘤细胞系中一致地减弱GPX4抑制剂诱导的脂质过氧化和细胞死亡,而它们对半胱氨酸运输抑制剂引发的铁下垂的抗性因细胞类型而异。这种对肿瘤的保护作用是通过巨噬细胞与肿瘤细胞接触和巨噬细胞衍生的细胞外囊泡(Mφ-EV)的传递介导的。转录组学和蛋白质组学分析显示,巨噬细胞和m - φ- ev增强了肿瘤细胞的谷胱甘肽代谢。值得注意的是,m- φ- ev独特地富含谷胱甘肽代谢相关蛋白PRDX6。从机制上讲,PRDX6的谷胱甘肽过氧化物酶活性提高细胞内还原性谷胱甘肽,抑制脂质过氧化,从而减轻铁凋亡。此外,巨噬细胞来源的PRDX6可减少线粒体超氧化物积累,减轻铁噬诱导的线粒体自噬,提高肿瘤细胞活力,最终促进肿瘤生长。总之,我们的研究结果提供了一种新的TME铁凋亡抵抗机制,其中巨噬细胞通过绕过GPX4抑制赋予肿瘤细胞弹性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
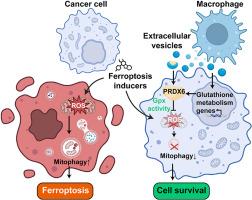
Macrophages and macrophage extracellular vesicles confer cancer ferroptosis resistance via PRDX6-mediated mitophagy inhibition
Ferroptosis has emerged as a promising therapeutic target in cancer therapy, with the tumor microenvironment (TME) playing a pivotal role in regulating ferroptosis. Although macrophages contribute to ferroptosis regulation within TME, the underlying mechanisms remain unclear. In this study, we demonstrate that macrophages consistently attenuate GPX4 inhibitor-induced lipid peroxidation and cell death in various tumor cell lines, whereas their resistance to cysteine transport inhibitor-triggered ferroptosis varies across cell types. This tumor protection from ferroptosis is mediated through macrophage-tumor cell contact and the delivery of macrophage-derived extracellular vesicles (Mφ-EV). Transcriptomic and proteomic analyses revealed that macrophages and Mφ-EV enhance glutathione metabolism in tumor cells. Notably, Mφ-EV are uniquely enriched with the glutathione metabolism-related protein PRDX6. Mechanistically, the glutathione peroxidase activity of PRDX6 elevates intracellular reduced glutathione, suppresses lipid peroxidation, and thereby mitigates ferroptosis. Furthermore, macrophage-derived PRDX6 reduces mitochondrial superoxide accumulation, alleviates ferroptosis-induced mitophagy, and enhances tumor cell viability, ultimately promoting tumor growth. Together, our findings provide a novel mechanism of ferroptosis resistance in TME, wherein macrophages confer tumor cell resilience by bypassing GPX4 inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


