小鼠断奶前后母体胰岛的转录组修饰。
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
确定增强β细胞质量的机制和分子因素对于制定对抗糖尿病的策略至关重要,因为β细胞质量随着疾病进展而下降。最近的研究表明,在小鼠断奶期间,β细胞增殖增加,胰岛显著扩张。本研究旨在确定与断奶前后胰岛变化相关的转录本,这是一个以前未在胰岛中探索过的生理阶段。在四个关键时间点对转录组进行核糖核酸(RNA)测序分析:哺乳结束时,β细胞增殖增加;断奶当天,激素和代谢环境从泌乳阶段过渡到非泌乳阶段;断奶后第三天,胰岛面积达到峰值,与我们之前的研究结果一致;在年龄匹配的雌性对照小鼠中。结果显示动态转录组变化。泌乳素调节基因的信使核糖核酸(mRNA)表达水平,包括其受体、信号抑制剂Cish、色氨酸羟化酶和骨保护素,在泌乳期间升高,随后下降。血浆催乳素浓度在哺乳期升高,但血浆骨保护素水平在各组间保持稳定。值得注意的是,未观察到已知的催乳素调节的细胞周期蛋白(如Ccna2、Ccnb1和Ccnb2)的变化。然而,Cdkn1a(细胞周期的负调节因子)的表达减少被注意到。令人惊讶的是,显微镜分析显示胰岛素免疫染色阴性的胰岛外周细胞凋亡标志物增加。这项研究首次确定了断奶前后的转录组学和细胞变化,为胰岛质量可塑性提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
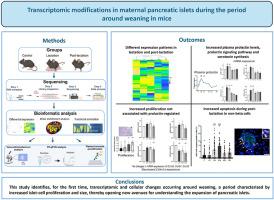
Transcriptomic modifications in maternal pancreatic islets during the period around weaning in mice
Identifying the mechanisms and molecular factors that enhance beta-cell mass is crucial for developing strategies to combat diabetes, as beta-cell mass declines with disease progression. Recent research has indicated an increase in beta-cell proliferation and a significant islet expansion around the weaning period in mice. This study aims to identify transcripts associated with changes in the islets around weaning—a physiological stage previously unexplored in islets. A ribonucleic acid (RNA) sequencing analysis of the transcriptome was performed at four key time points: the end of lactation, when beta-cell proliferation increases; the day of weaning, when the hormonal and metabolic environment transitions from lactation to the non-lactating stage; the third day post-weaning, when islet area peaks, as observed in our prior studies; and in age-matched female control mice. The results revealed dynamic transcriptomic changes. The messenger ribonucleic acid (mRNA) expression levels of genes regulated by prolactin, including its receptor, signaling inhibitor Cish, tryptophan hydroxylase, and osteoprotegerin, increased during lactation and subsequently declined. Plasma prolactin concentrations rose during lactation, but plasma osteoprotegerin levels remained stable across groups. Notably, no changes were observed in known prolactin-regulated cyclins that positively influence the cell cycle, such as Ccna2, Ccnb1, and Ccnb2. However, a decrease in the expression of Cdkn1a, a negative regulator of the cell cycle, was noted. Surprisingly, microscopy analysis indicated increased apoptosis markers in islet peripheral cells that were negative for insulin immunostaining. This study is the first to identify transcriptomic and cellular changes around weaning, offering new insights into islet mass plasticity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


